Carbon Nanomaterials for energy harvest, storage and conversion
 A
method has been developed in the group to selectively control the
graphene orientations towards the axis of the carbon nanofibers. The
principle for rationally design the nanostructure of CNFs has been
gained by the detailed kinetic study and characterization of
resulted CNFs. Carbon nanotubes, herringbone nanofibers, platelet
nanofibers, onions and graphene are examples among the carbon
nanomaterials. They provide a platform for study effects of graphene
orientations on the properties of different carbon nanomaterials in
different applicators. Synthesis of aligned CNTs on metallic
substrates and graphene are the main topics in the group with an aim
of direct application as energy conversion and storage devices.
A
method has been developed in the group to selectively control the
graphene orientations towards the axis of the carbon nanofibers. The
principle for rationally design the nanostructure of CNFs has been
gained by the detailed kinetic study and characterization of
resulted CNFs. Carbon nanotubes, herringbone nanofibers, platelet
nanofibers, onions and graphene are examples among the carbon
nanomaterials. They provide a platform for study effects of graphene
orientations on the properties of different carbon nanomaterials in
different applicators. Synthesis of aligned CNTs on metallic
substrates and graphene are the main topics in the group with an aim
of direct application as energy conversion and storage devices.
 CNFs have unique properties such as high cystallinity, tuneable
surface structure and surface groups, making them promising directly
as catalysts for oxidation or oxidative dehydrogenation. The
research has been focused on effects of graphene orientations of
CNFs on oxidative dehydrogenation of propane and ethyl benzene.
CNFs have unique properties such as high cystallinity, tuneable
surface structure and surface groups, making them promising directly
as catalysts for oxidation or oxidative dehydrogenation. The
research has been focused on effects of graphene orientations of
CNFs on oxidative dehydrogenation of propane and ethyl benzene.
 The edge structures, which as governed by the graphene sheet
orientations of CNFs, significantly influence the interaction
between the metal nanoparticles and CNFs. This directly determinates
the metal cluster shapes, and thus the bond length distribution and
facets exposed to the reactants. The reactive force field molecular
dynamic simulations is a powerful tool to understand the interaction
between nanoclusters and CNFs. The CNFs with different graphene
orientations have been developed as a platform for rational catalyst
design to manipulate the interaction, electronic properties and
particle shapes. The CNFs supported Ni, Co, Cu, Pd, Pt and Ru have
been studied in both gas phase and liquid phase reactions.
The edge structures, which as governed by the graphene sheet
orientations of CNFs, significantly influence the interaction
between the metal nanoparticles and CNFs. This directly determinates
the metal cluster shapes, and thus the bond length distribution and
facets exposed to the reactants. The reactive force field molecular
dynamic simulations is a powerful tool to understand the interaction
between nanoclusters and CNFs. The CNFs with different graphene
orientations have been developed as a platform for rational catalyst
design to manipulate the interaction, electronic properties and
particle shapes. The CNFs supported Ni, Co, Cu, Pd, Pt and Ru have
been studied in both gas phase and liquid phase reactions.
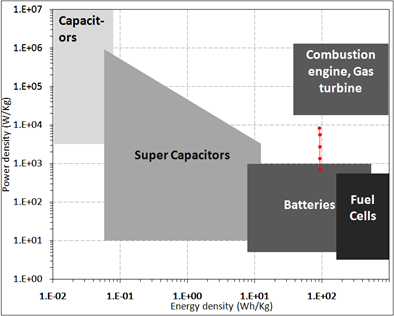
 There
is an increasing demand for energy storage devices with high power
ad energy density for applications
in renewable energy production, electric or hybrid cars, etc..
Supercapacitors with hybrid electrodes will share an important role
in energy storage market. An important development in energy storage
is to move the boundary of energy density of supercapacitors towards
to batteries, as shown in Ragona plot. Asymmetric hybrids combine
Faradaic and non-Faradaic processes by coupling an carbon electrode
with a pseudocapacitor electrode through redox reactions. In
particular, the coupling of a CNT negative electrode with a
conducting polymer positive electrode has received a great deal of
attention.
There
is an increasing demand for energy storage devices with high power
ad energy density for applications
in renewable energy production, electric or hybrid cars, etc..
Supercapacitors with hybrid electrodes will share an important role
in energy storage market. An important development in energy storage
is to move the boundary of energy density of supercapacitors towards
to batteries, as shown in Ragona plot. Asymmetric hybrids combine
Faradaic and non-Faradaic processes by coupling an carbon electrode
with a pseudocapacitor electrode through redox reactions. In
particular, the coupling of a CNT negative electrode with a
conducting polymer positive electrode has received a great deal of
attention.
 The
research has focused on increasing the power and energy density of
the supercapacitors, which is closely linked to the following
objectives: 1) Design 3-D pillar structure integrating CNT with
conductive polymer to have high surface area of conductive polymer
with tailored thickness and regular open structure, aiming at a
better access of electrolyte. 2) Synthesis of CNT arrays on Al foils
aiming at reducing the weight of electrode and reduced resistance.
3) Electrochemical in-situ polymerization of conductive polymers
with tailored thickness. 4) Fundamental study of the relationship
among the conductive thickness, electric resistance, ionic
transport, capacitance, stability, energy and power density. 5)
Fundamental study of the interface properties between the electrode
surfaces and different electrolytes to increase the energy density.
Prototype supercapacitors have been fabricated. The supercapacitors
presented a high energy density (almost 2 order of magnitudes higher
than conventional carbon based capacitors) and high power density
(1-2 order of magnitudes higher than batteries. Similar structure of
electrodes with different electroactive materials are testing in
Li-ion batteries.
The
research has focused on increasing the power and energy density of
the supercapacitors, which is closely linked to the following
objectives: 1) Design 3-D pillar structure integrating CNT with
conductive polymer to have high surface area of conductive polymer
with tailored thickness and regular open structure, aiming at a
better access of electrolyte. 2) Synthesis of CNT arrays on Al foils
aiming at reducing the weight of electrode and reduced resistance.
3) Electrochemical in-situ polymerization of conductive polymers
with tailored thickness. 4) Fundamental study of the relationship
among the conductive thickness, electric resistance, ionic
transport, capacitance, stability, energy and power density. 5)
Fundamental study of the interface properties between the electrode
surfaces and different electrolytes to increase the energy density.
Prototype supercapacitors have been fabricated. The supercapacitors
presented a high energy density (almost 2 order of magnitudes higher
than conventional carbon based capacitors) and high power density
(1-2 order of magnitudes higher than batteries. Similar structure of
electrodes with different electroactive materials are testing in
Li-ion batteries.
 High
conductivity, high external surface, tuneable surface structure and
properties make carbon nanofibers promising as fuel cell catalyst
supports. Our research was a part of the national project on
nanomaterials for hydrogen conversion supported by Norwegian
Research Council.
The objectives are to gain a better understanding of the following
effects on the fuel cell catalyst properties:
1) Nano carbon structure, in particular the greaphene orientations;
2) Interaction between CNF and Pt particles; 3) Pt particle sizes
and shapes;
4) Surface oxygen groups
High
conductivity, high external surface, tuneable surface structure and
properties make carbon nanofibers promising as fuel cell catalyst
supports. Our research was a part of the national project on
nanomaterials for hydrogen conversion supported by Norwegian
Research Council.
The objectives are to gain a better understanding of the following
effects on the fuel cell catalyst properties:
1) Nano carbon structure, in particular the greaphene orientations;
2) Interaction between CNF and Pt particles; 3) Pt particle sizes
and shapes;
4) Surface oxygen groups
 The
structured CNFs, for examples, CNF/carbon felt and CNF/monoliths can
be used to protein separation and waster water treatment. The
adsorptive removal of phenol and reactive removal N containing
pollutants in water are the research topics. An EU project is
running on this topic.
The
structured CNFs, for examples, CNF/carbon felt and CNF/monoliths can
be used to protein separation and waster water treatment. The
adsorptive removal of phenol and reactive removal N containing
pollutants in water are the research topics. An EU project is
running on this topic.
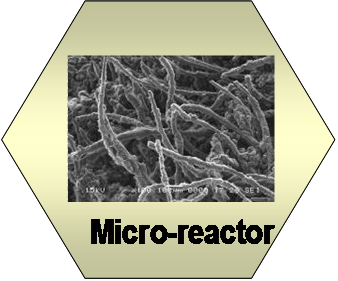 Hierarchically
structured CNFs on carbon felt, monolith, foils and foams can be
used as microreactors to facilitate mass and heat transfer in the
reactor, and achieve almost 100% effectiveness of nanoclusters in
the reactor. The structured reactors are highly conductive, which is
suitable for highly exothermic reactions such as CO oxidation and
Fischer-Tropsch reactions. In addition, the structures reactor
itself can be used as a heating element to rapid heat up the
reactor. Recent a study illustrated that the CNF/carbon felt
composite can avoid running away and achieve uniform distribution of
temperature in the reactor and achieve 100% effectiveness of Co
nanoclusters.
Hierarchically
structured CNFs on carbon felt, monolith, foils and foams can be
used as microreactors to facilitate mass and heat transfer in the
reactor, and achieve almost 100% effectiveness of nanoclusters in
the reactor. The structured reactors are highly conductive, which is
suitable for highly exothermic reactions such as CO oxidation and
Fischer-Tropsch reactions. In addition, the structures reactor
itself can be used as a heating element to rapid heat up the
reactor. Recent a study illustrated that the CNF/carbon felt
composite can avoid running away and achieve uniform distribution of
temperature in the reactor and achieve 100% effectiveness of Co
nanoclusters.
