Hydrogen production by sorption enhanced reactions
 A
soft chemical route has been developed to synthesize nanocrystllite
solid mixed oxides as high temperature CO2 acceptors,
such as Li2ZrO3, Na2ZrO3
and CaO based mixed oxides. The main objective is to gain better
kinetics, selectivity and stability in the presence of steam.
Research focus has been on the principles for rational design of the
nanomateraisl in terms of backbone structure, crystal size of active
materials and morphology of the particles.
A
soft chemical route has been developed to synthesize nanocrystllite
solid mixed oxides as high temperature CO2 acceptors,
such as Li2ZrO3, Na2ZrO3
and CaO based mixed oxides. The main objective is to gain better
kinetics, selectivity and stability in the presence of steam.
Research focus has been on the principles for rational design of the
nanomateraisl in terms of backbone structure, crystal size of active
materials and morphology of the particles.
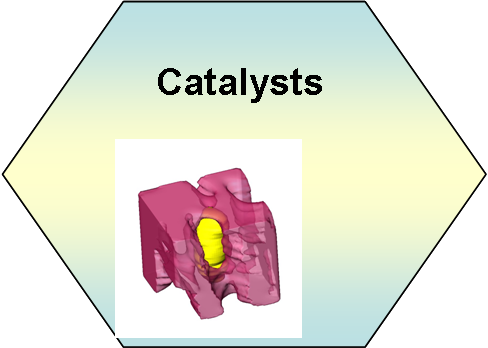 Research
is focusing on hydrotalcite derived transition metal catalysts, such
as Ni, Ni-Co catalysts. Detailed kinetic study and characterization
such as 3D electron tomography are used to investigate the
structures and properties on metal clusters in the support matrix.
The objective is to improve the activity of metal cluster towards
C-C and C-H bond activation and thermal stability.
Research
is focusing on hydrotalcite derived transition metal catalysts, such
as Ni, Ni-Co catalysts. Detailed kinetic study and characterization
such as 3D electron tomography are used to investigate the
structures and properties on metal clusters in the support matrix.
The objective is to improve the activity of metal cluster towards
C-C and C-H bond activation and thermal stability.
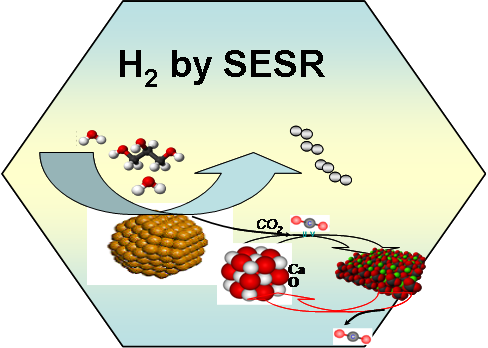 Sorption
enhanced steam reforming (SESR) has been studied fro enhanced
hydrogen production form methane, hydrocarbons and biomass derived
oxygenates such as ethanol, glycerol, sorbitol, glucose, crude
glycerol and synthesis gas form biomass gasification. The catalysts
and CO2 acceptors are installed together in the twin
fixed bed reactors, one for SESR and one for regeneration of CO2
acceptors. The in-situ removal of CO2 shifts equilibrium
towards hydrogen production. Very high purity of hydrogen (>99%) can
be produced by SESR reactions. The research is aiming to develop a
complete tool box for conversion of biomass derived diverse mono or
multifunctional oxygenates including bio-oil to hydrogen with high
energy efficiency.
Sorption
enhanced steam reforming (SESR) has been studied fro enhanced
hydrogen production form methane, hydrocarbons and biomass derived
oxygenates such as ethanol, glycerol, sorbitol, glucose, crude
glycerol and synthesis gas form biomass gasification. The catalysts
and CO2 acceptors are installed together in the twin
fixed bed reactors, one for SESR and one for regeneration of CO2
acceptors. The in-situ removal of CO2 shifts equilibrium
towards hydrogen production. Very high purity of hydrogen (>99%) can
be produced by SESR reactions. The research is aiming to develop a
complete tool box for conversion of biomass derived diverse mono or
multifunctional oxygenates including bio-oil to hydrogen with high
energy efficiency.
 High
purity hydrogen can be produced by sorption enhanced catalytic
gasification of wood biomass powders. The presence of catalysts
enhances the conversion of tars formed by biomass pyrolysis. The
in-situ removal of CO2 enhances further the tar
conversion and steam reforming of oxygenates and hydrocarbons to
hydrogen. The research goal is to formulate the CO2
acceptors, catalysts and operating conditions to reach a high energy
efficiency and high hydrogen yield as we as purity.
High
purity hydrogen can be produced by sorption enhanced catalytic
gasification of wood biomass powders. The presence of catalysts
enhances the conversion of tars formed by biomass pyrolysis. The
in-situ removal of CO2 enhances further the tar
conversion and steam reforming of oxygenates and hydrocarbons to
hydrogen. The research goal is to formulate the CO2
acceptors, catalysts and operating conditions to reach a high energy
efficiency and high hydrogen yield as we as purity.
 Understanding
of reaction mechanism and kinetic modeling of carbonation reactions of basic
oxides is one of the research topics. Detailed kinetic study is performed in
terms of CO2 concentration, temperature and steam concentration. The
reactor modeling and simulation of SESR in fixed bed and fluidized bed reactors
are cooperated with reactor group at NTNU.
Understanding
of reaction mechanism and kinetic modeling of carbonation reactions of basic
oxides is one of the research topics. Detailed kinetic study is performed in
terms of CO2 concentration, temperature and steam concentration. The
reactor modeling and simulation of SESR in fixed bed and fluidized bed reactors
are cooperated with reactor group at NTNU.
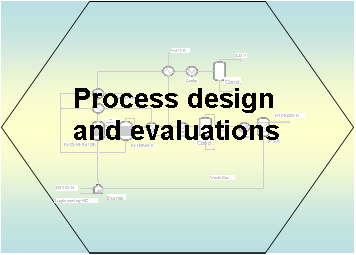 Thermodynamic
analysis is performed to predict the maximum hydrogen purity and
yield in SESR of different molecules. The SESR are integrated into
the process by taking into account the integration of carbonation
and decarbonation reactions, the heat integration in the process
design. The hydrogen yield and energy efficiency are evaluated for
the different design. Economic evaluation is also performed for
selected processes.
Thermodynamic
analysis is performed to predict the maximum hydrogen purity and
yield in SESR of different molecules. The SESR are integrated into
the process by taking into account the integration of carbonation
and decarbonation reactions, the heat integration in the process
design. The hydrogen yield and energy efficiency are evaluated for
the different design. Economic evaluation is also performed for
selected processes.
