Compounds:
Se-anchored
carotenoid derivatives
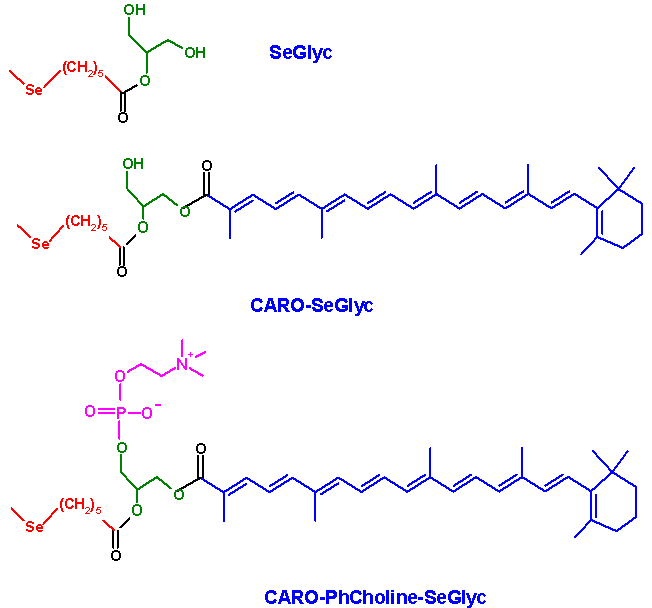
Caro-Glyc-Se
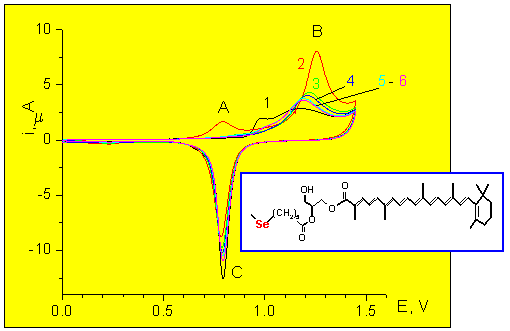
Anodic reactions
Electrolyte: 0.01 M HClO4
Presented at the 36th Heyrovský Discussion, 2003
In cooperation with Bente-Jeanette Foss, Ana Ion, Vassilia Partali and Richard Sliwka
Details: Bente-Jeanette Foss, Ana Ion, Vassilia Partali, H.-R.Sliwka, and F. G. Banica, ”Electrochemical and EQCM investigation of a selenium derivatized carotenoid in the self-assembled state at a gold electrode”, J. Electroanal. Chem., 593 (2006) 15-28. Full paper ; Bente Jeanette Foss, Ana Ion, Vassilia Partali, H -R Sliwka and F. G. Banica, "1-(7-Selenaoctanoyl)-glycerol as an Anchor for Self-Assembly of Biological Compounds at the Gold Surface", Collect. Czech. Chem. Commun.,69 ( 2004) 1971-1996. Abstract
Background
Selenium-derivatizes carotenoids were adsorbed to the gold electrode surface via the Se function.The electrochemical behavior of the surface layer was thereafter investigated in an aqueous solution.
Se-anchored
carotenoid derivatives

Anodic reactions
Electrolyte: 0.01 M HClO4

Peak B: Anodic desorption + Au oxidation
Peak C: AuO reduction
Curve 1: Plain gold
Curves 2- 6: Succesive
scans at the modified electrode
CARO-Glyc-Se: Effect of the modification time
Modifier: 4 mM in acetonitrile. CV runs in 0.01 M HClO4
Curves 1 and 2: Charge (Q) for:
1. Anodic reaction of the
carotenoid fragment (peak A)
2. Anodic desorption (peak
B, corrected for Au oxidation)
Curve 3: AC current at +0.10 V (250 Hz, 10 mV rms)
CARO-Glyc-Se: Anodic reaction of the CARO fragment
Electrolyte: 0.01 M HClO4
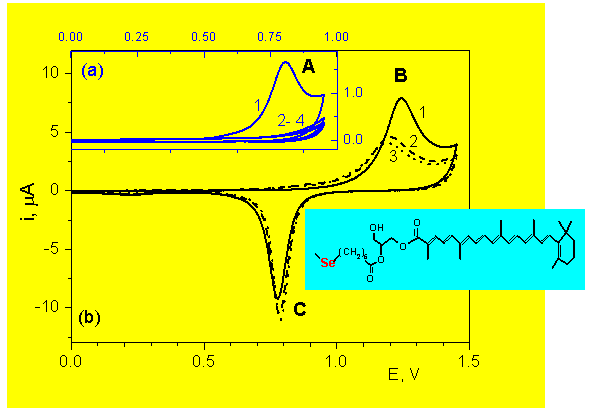
A: Carotenoid anodic reaction
B: Anodic desorption + Au oxidation
C: AuO reduction
1 - 4: Succesive scans
CARO-Glyc-Se
Effect of the anodic reaction (peak A)
on the anodic desorption process (peak B)
Electrolyte: 0.01 M HClO4
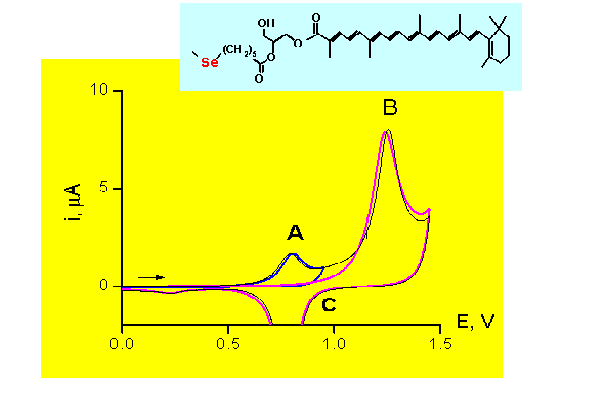
Conclusion: Peak A reaction has no effect on the anodic desorption process
CARO-Glyc-Se
FRA for (1) a freshly modified electrode and
(3) after performing the anodic reaction (curve 2, peak A)
Fe(CN)63-/4-
(5 mM each) in 1 M KNO3
Conclusion:q
>
0.995 and increses after performing peak A reaction
CARO-Glyc-Se: Cathodic reactions
0.5 M KOH; scan rate 100 mV/s

CARO-Glyc-Se: Cathodic reactions
A test for the occurrence of the cathodic desorption
Q1 = Charge for anodic desorption; Q2 = Charge for gold oxide reduction
|
|
|
|
|
|
| 1. CV scan in the anodic region |
|
|
|
|
| 2.(i) CV scan in the cathodic region, followed by (ii) a CV scan as in (1) |
|
|
|
|
| 3. (i) Anodic oxidation of the carotenoid fragment (peak A), followed by (ii) a CV scan in the cathodic range and (iii) a CV scan as in (1) |
|
|
|
Conclusion: no cathodic desorption occurs