A summary of recent results
The outstanding importance of amino acids as building blocks of proteins is well known but many amino acids enjoy a particular attention due to specific health and nutritional issues. Seleno-methionine (SeMet, inset to Fig. 1) which belongs to this category is a selenium analogue of the essential amino acid methionine and is widely used as a nutritional supplement in order to correct for selenium deficiency in humans and livestock. We decided to investigate this compound [1 ], [2 ] not only because of its biological relevance but also because it displays some interesting features from the standpoint of molecular electronics. Indeed, selenium functionalities display a strong affinity to metals like gold and promote the adsorption of organic derivatives to this metal surface. In this form, the selenium functionality is an efficient electron bridge between a metal and a conducting molecule.
We have previously investigated the reactivity of organic monoselenides (including an electron acceptor moiety) in an adsorbed state on gold [ 3 ], [4 ]. Compared to these compounds, SeMet has a less intricate structure that provides evidence of characteristic features of monoselenide functionality through interactions with a metal surface. Moreover, adsorbed SeMet is relevant to the study of the effects of charged interfaces on the ionization of amino acid functionalities.
The
interaction of SeMet with the charged gold surface, as well as the reactivity
of the adsorbed species in contact with aqueous solutions, was studied
by electrochemical methods (including electrochemical impedance spectrometry)
and by the piezoelectric nano-balance.
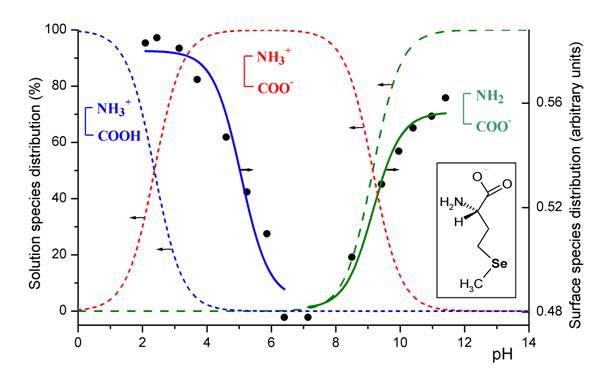 Fig. 1. Ionization of the amino acid functions in the seleno-methionine molecule adsorbed at a polycrystalline gold surface (solid lines) as compared with the similar process occurring in the solution phase (broken lines). Blue: carboxyl ionization; green: ammonium ionization. |
An examination of electric charge distribution at the interface revealed the ionization state of the adsorbed amino acid molecule as a function of solution pH. Relevant results are presented in Fig. 1 which displays the distribution of protonation states for the dissolved (broken lines) and adsorbed (solid line) form. From this it is clear, that the adsorption induces a marked decrease in the acidic strength of the carboxyl group whereas the ionization of the amino group experiences negligible modifications under these conditions. The modification of the acidity is the result of the interplay between various factors like surface electric charge, hydrogen bonding and electrostatic interactions. These results demonstrate for the first time the possibility of tuning the ionization constant of an amino acid by a physical process: adsorption at a charged metal. Up till now, chemical derivatization that involves a significant alteration of the molecule configuration was the only way for achieving this goal. Self-assembly of amino acids by chemisorption at metal surfaces can therefore be a viable approach that contributes to the field of artificial enzymes [ 5 ] since modifying the acid strength of the amino acid residue could imparts catalytic activity to the system.
The reactivity of the selenium group was mainly established by means of the piezoelectric nano-balance in conjunction with electrochemical determinations. It was observed that adsorption causes partial conversion to seleno-homocysteine of SeMet by cleaving the methyl selenium group. The stoichiometry of the electrochemical oxidation processes involving the adsorbed compounds was also assessed and elemental selenium as well as different oxygenated organic selenium derivatives has been identified as oxidation products.
Another feature of interest is the metal ion binding to adsorbed amino acids and its effects on the electrochemical reactivity of the metal ion. We have demonstrated that SeMet can act as a catalyst in nickel ion reduction [ 2 ] and this property was applied to develop an analytical method for determining SeMet in nutritional supplements. According to the manufacturer´s guidelines, selenium can be present in such products either as an organic (SeMet) or inorganic compound (sodium selenite or selenious acid). Our method can reliably detect SeMet without interference from various forms of inorganic selenium. Further investigations proved that analogous methods are suitable for determining other amino acids of nutritional relevance such as arginine and ornithine [6 ].
In conclusion, amino acid adsorption to metal surface via side-chain functionality may modify the acid-base behavior of the specific amino acid groups with potential applications in controlling the reactivity of such compounds. Our continuing studies are aimed at exploring in more detail the effect of the surface electric charge and surface ion distribution in order to design a procedure for gradually tuning the acid properties of the adsorbed amino acid. Photoelectron spectrometry will be employed for investigating the interaction of the binding group with the gold substrate.
References
[ 1
] Ana Banica, Alina Culetu, F.G. Banica, J. Electroanal. Chem.,
599 (2007) 100.
[2
] F. G. Banica, B. Kafar, S. Skrzypek, W. Ciesielski, Electroanalysis,
18 (2006) 2269.
[3
] B.-J. Foss, A. Ion, V. Partali, H.-R.Sliwka, F. G. Banica, J. Electroanal. Chem.,
593 (2006) 15-28.
[ 4
] B.-J. Foss, A. Ion, V. Partali, H.-R Sliwka, F. G. Banica, Collect.
Czech. Chem. Commun., 69 (2004) 1971.
[ 5
] Y.
Murakami, J. Kikuchi, Y. Hisaeda, O. Hayashida, Chem. Rev.
96 (1996) 721.
[ 6
] F. G. Banica, D. Guziejewski, S. Skrzypek,
W. Ciesielski, Bioelectrochemistry, manuscript submitted in revised
form.