108g Performance Enhancement in a Direct Methanol Fuel Cell Via an Externally Applied Oscillatory Flow
In this work, an oscillatory stroke volume was applied to the anode flow channel of a 5 cm2 DMFC with a standard membrane electrode assembly (anode: 4.0 mg/cm2 50:50 Pt:Ru, cathode: 2.0 mg/cm2 Pt, membrane: Nafion 117) and a diffusion layer of Toray carbon fiber paper (TGP-H-060). This introduced a time-dependent component to the steady flow in the anode channels. Over one full period of oscillation, the time-averaged flow rate remains the same, but additional convective motion is supplied while the heat transfer difficulties normally encountered when using large steady flow rates are avoided. The voltage-current density relationship was measured over a range of stroke volumes (0.03 – 0.79 mL) and oscillation frequencies (0.5 – 6.2 Hz) and compared to measurements taken at identical operating conditions (anode steady flow rate = 12 mL/min, T = 70 °C, [MeOH] = 1.0 M) without the oscillatory motion. For smaller stroke volumes and frequencies, no appreciable change from normal operation was observed. For larger stroke volumes and frequencies, the limiting current density increased significantly; values as high as an approximately twofold improvement were measured. The peak power density was also enhanced by as much as 25%. The theoretical energy cost of applying oscillatory flow to the DMFC anode is only a fraction (10% or less) of the power that was gained.
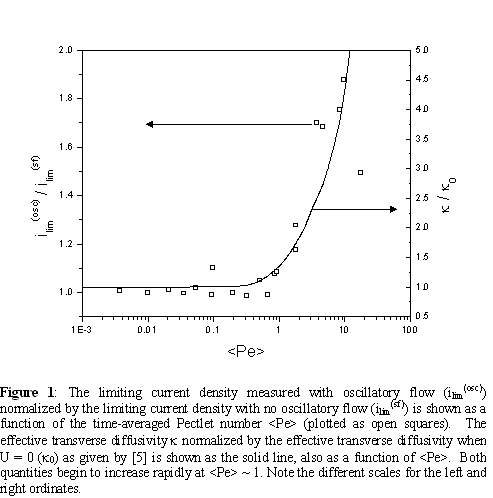
The pressure gradients generated by the oscillatory stroke volume were significant enough to penetrate into the diffusion layer of the anode and induce a Darcy's law flow parallel to the catalyst surface. To our knowledge, the mass transfer problem governing the value of the effective transverse diffusivity (DT) when oscillatory flow is induced in a porous media is unsolved. However, the amplitude of the oscillatory motion in the flow-direction (Δx) is much greater than the radii of the carbon fibers (a) that compose the anode's diffusion layer. It was found that at all experimental conditions, values of Δx/a are sufficient to justify apply existing steady flow solutions to the oscillatory flow problem. Steady flow theory of dispersion in random porous systems [4,5] predicts that DT ~ λUa when the Pectlet number (Pe) = Ua/Do >> 1, where U is an average velocity through the porous media, Do is the molecular diffusivity of the solute and λ is an O(1) constant which may be a function of porosity and other geometric quantities. When oscillatory flow experiments are compared to the steady flow base case, significant enhancement in the limiting current density is observed to begin when the time-averaged Pectlet number <Pe> ~ O(1), which corresponds with the point at which DT becomes large with respect to Do, thereby reducing the mass transfer limitations at the anode (Figure 1). This evidence suggests the mass transfer limitations in a DMFC anode are diffusive in nature. Modifications to the DMFC anode designed to take maximum advantage of this mass transfer improvement and consequently, cell performance, will be discussed.
References
[1] Scott, K.; Taama, W.M.; Kramer, S.; Argyropoulos, P.; Sundmacher, K. Electrochimica Acta 45, 945–957, 1999.
[2] Schultz, T.; Zhou, S.; Sundmacher, K. Chem. Eng. Tech., 24 (12), 1223–1233, 2001.
[3] Nordlund, J.; Roessler, A.; Lindbergh, G. J. Appl. Electrochem. 32, 259–265, 2002.
[4] Koch, D.L.; Brady, J.F. J. Fluid Mech. 154, 399–427, 1985.
[5] Koch, D.L.; Brady, J.F. AIChE J. 32 (4), 575–591, 1986.