488f Separation of Azeotropic Alcohol-Water Mixtures with Fricdiff
Separation of azeotropic alcohol-water mixtures with FricDiff
Authors: B. Breure (B.Breure@tue.nl), E.A.J.F. Peters (E.A.J.F. Peters@tue.nl), P.J.A.M. Kerkhof (P.J.A.M.Kerhof@tue.nl)
Address: TU Eindhoven, Laboratory of Separation Processes and Transport Phenomena, Den Dolech 2, 5612 AZ
Telephone: +31 40 247 3683
Keywords: FricDiff, kinetic separation, azeotropic alcohol-water mixtures, enhancer, numerical model
One of the major disadvantages of the separation processes that are currently used in the chemical and petro-chemical industry is their high energy-consumption. This is particularly the case for distillation. Especially when separation processes become more complex, for example in the case of the separation of azeotropic mixtures, energy-demands are high. In addition, hazardous chemicals are often required to facilitate the separation.
Over the past years, the interest for membrane separation processes, like pervaporation and vapor permeation has grown tremendously. The relatively low energy consumption and the fact that no additional chemicals are required, makes these processes interesting candidates to replace, among others, distillative separations. In spite of major efforts in universities and research institutes, membrane processes are still not suited to be applied on a large scale in the chemical and petro-chemical industry. One of the reasons for this is that the fluxes through the membranes are low, implying that large membrane areas are required. Furthermore, the membranes that are used in these processes give rise to problems. Membrane modules equipped with ceramic membranes are very expensive and it is difficult to synthesize defect-free ceramic membranes. Polymeric membranes can also be used, but they have a low chemical and thermal stability.
At our laboratory we are developing a separation process that doesn't have these disadvantages. This separation process, which is named FricDiff ([1]
A typical FricDiff module consists of two compartments separated by a non-selective macro-porous barrier (macro-porous membrane). Through the compartments the feed mixture and the enhancer are flowing, co-currently or counter-currently. The separation process mainly takes place inside the pores of the barrier. The main function of the barrier is to prevent mixing of the feed mixture and the enhancer gas or vapor. Furthermore, the barrier decreases convective mass transport through the barrier as a result of possible pressure differences over the barrier. The advantage of using a macro porous barrier instead of a selective micro porous membrane is that the fluxes through the barrier are much higher. In addition, the barrier can be made of a robust and relatively cheap material, like stainless steel.
In this project, the applicability of FricDiff for the separation of azeotropic mixtures of alcohols and water is studied. Experiments are performed with a lab scale experimental set-up with various alcohol-water mixtures. The experimental data are compared with results obtained with a detailed numerical model developed in the finite element program Comsol MultiphysicsTM. The separation process in the FricDiff-module is described by simultaneously solving mass and momentum balances in the porous barrier and in the flow channels where the feed mixture and the enhancer are flowing. The mass transport through the porous barrier is described with an advanced model for transport through porous media: the Binary Friction Model [7]
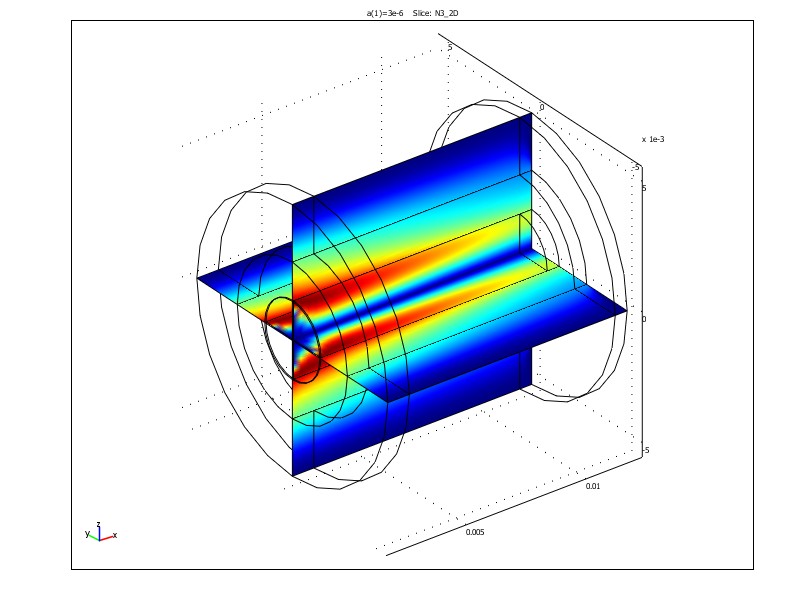
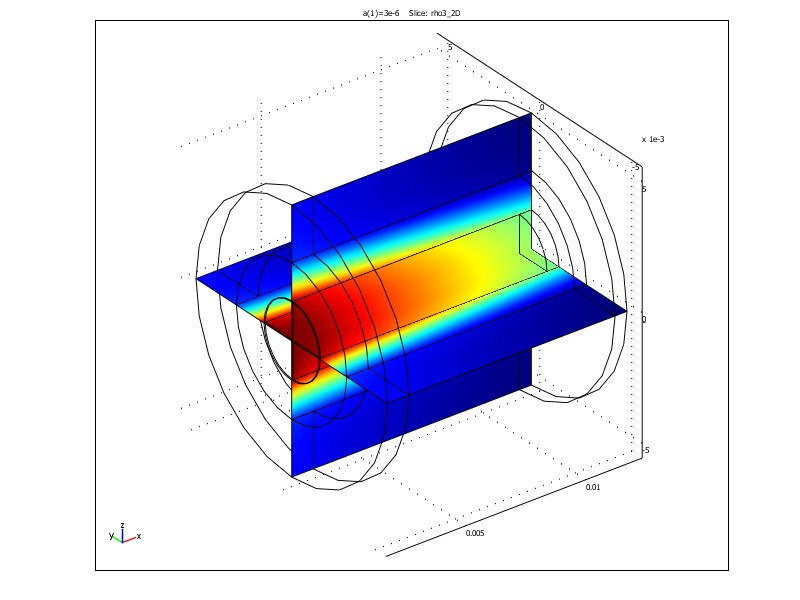
Figure 1. Radial fluxes of IPA (left) and concentration distribution of IPA (right) in a tubular FricDiff-module. Results are obtained in the finite element program Comsol MultiphysicsTM.
The influence of process conditions (e.g. absolute pressure, ratio of flow rates of feed mixture to enhancer), the geometry of the module (tubular or plate configuration) and characteristics of the porous barrier (e.g. pore size, thickness of the porous barrier) on the separation are studied both experimentally and numerically. Attention is also focused on the negative influences that concentration polarization and concentration boundary layers can have on the separation process in the module. Concentration polarization is a phenomenon that occurs in all membrane separation processes and it adversely affects the separation performance of the module. It reduces the driving force for mass transfer across the membrane and hence is a phenomenon that one wants to prevent from occurring. For this reason the impact of concentration polarization on the process performance of a FricDiff-module under various operating conditions is studied. It will be shown that with decreasing thickness of the porous barrier resistances in the boundary layer adjacent to the barrier start to become important. Therefore, their influence on the separation process has to be taken into account.
By performing the above-mentioned analysis, a set of criteria is defined that can be used to optimize the FricDiff separation process for the separation of a specific mixture.
[2] B. Breure, E.A.J.F. Peters, P.J.A.M. Kerkhof, Separation of azeotropic mixtures of alcohols and water with FricDiff, Sep. Pur. Techn., accepted for publication (2008)
[5] M.T. Cichellli, W.D. Weatherford, J.R. Bowman, Sweep diffusion gas separation process, Part I, Chem. Eng. Prog. 47(2) 63-74 (1951)