615a Interaction of Microwave Heating with Heterogeneous Organometallic Systems: Application to the Grignard and Reformatsky Reaction
Higher conversion rates observed for certain organic reactions when heated by microwave irradiation compared to conventional heating techniques (referred to as a positive microwave effect) have attracted increasing attention of organic chemists over the last decade.
Although microwave heating (MW) accelerates certain reactions, applying it to large scale processes is not trivial. Accompanying investments, physical limitations (e.g. penetration depth of the irradiation, incompatibility of materials) and relatively high energy costs have to be balanced by benefits in production capacity and/or improved selectivity towards the desired product, improved safety during operation or more environmentally benign processes to justify application of MW on large scale. Although MW is not a universally beneficial technique applicable to all reactions it may expand the toolbox for process scale-up in the future. Comparing the limitations and advantages of MW gives insight into the usefulness on larger scale, and suggests that every reaction should be separately evaluated to select MW as a suitable tool.
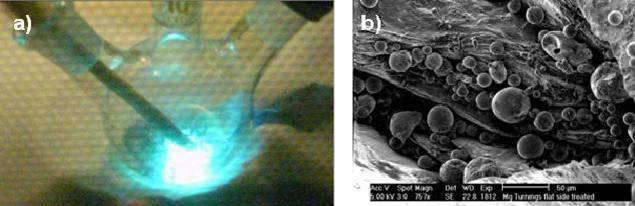
Figure 1: a) Violent electrostatic discharges induced by microwave irradiation b) SEM image of surface defects and spherical particles on the magnesium surface after microwave treatment
Recently we reported[1] that heterogeneous reactions are most likely to show a positive microwave effect. Also metal-catalyzed or -mediated reactions —heterogeneous and homogeneous— are reported as good candidates for microwave chemistry. We selected two classical examples to investigate the influence of microwave heating on the formation of reactive organometallic intermediates. The formation of organic magnesium halides (i.e. Grignard reagents) and zinc α-halo-esters (i.e. Reformatsky reagents) under conventional and MW conditions was compared in detail. Microwave irradiation causes violent electrostatic discharges on both metal surfaces (Figure 1a) strongly influencing initiation times of these reactions. Surprisingly the initiation time of the Grignard reagent formation reaction is reduced while the Reformatsky reagent formation is inhibited. Also the formation of organometallic compounds from less reactive chloride precursors can be accomplished without the necessity of chemical initiators. The elimination of initiators leads to a cleaner and safer reaction and a better reproducibility. The mechanistic details of the reduction in initiation time were studied by surface characterization of the metal (Figure 1b). The consequences for upscaling this type of microwave-accelerated processes are evaluated. The low penetration depth of the irradiation calls for the use of a batch loop reactor. In this setup only a relatively small, continuously flushed chamber is irradiated, circumventing the problems commonly associated with microwave heating. Although the electrostatic discharges can pose a safety issue it guarantees constant initiation times thus rendering it a viable candidate as heating technique for large scale synthesis.