188v Fluorescent Gold Nanoparticle for Bio-Imaging
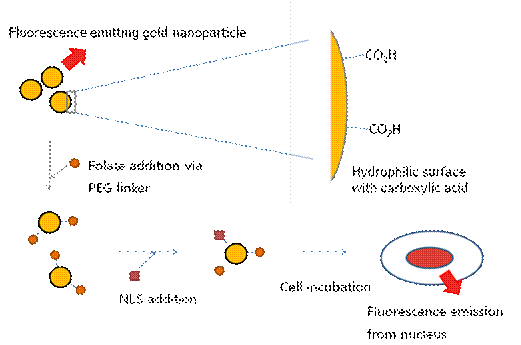
Water soluble fluorescent gold nanoparticles have been developed for replacing commercial dye molecules and toxic quantum dots in many applications including target specific bio-imaging agents. Except for a few specialized organic molecules whose toxicity profiles are yet to be explored, this fluorescence gold nanoparticle can be the most promising material so far. It is smaller than 5 nm in size, less toxic than their CdSe counterparts and also has much less photo-bleaching property comparing to commercial dyes. Recipes to prepare gold quantum dots trapped in dendrimers to make them bright and water soluble have been reported [1]. We are developing an alternative strategy to make water soluble gold quantum dots, using thiol based capping molecules (Figure 1). This approach has the advantage of producing relatively monodispersed gold nanoparticles, with tailorable solubility, and which is covered with functionalizable carboxylic groups on the surface for the attachment of peptides, DNA, and/or other signal, therapeutic molecules.
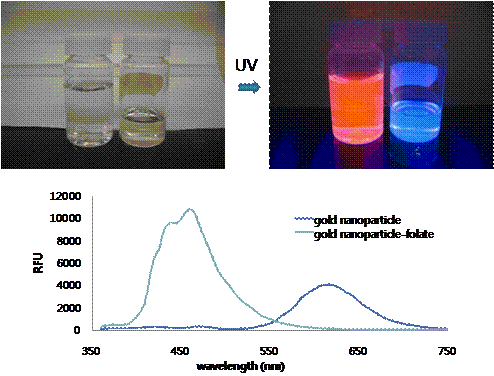
Figure 1. Scheme of gold nanoparticle for bio-imaging In this work, we studied the emission spectra of gold quantum dots prepared using mercaptooctanoic acid. It has thiol functional groups to bind and stabilize gold surface and carboxylic acid groups on the other side for the anchoring point to the conjugation of other molecules and also provide water solubility of the particles. The TEM analysis showed the average size of the gold nanoparticle is in range of 1 ~ 5 nm in diameter. Large Stoke shift, with UV excitation and 620 nm emission, is monitored and it is one of the unique features for this gold nanoparticle. It is possible that the meta-stable state orbital has formed in between the nanoparticles HOMO and LUMO orbitals to lower the emission energy which eventually results far red-shifted emissions. The intensity of the emission is linearly proportional to the concentrations of the gold nanoparticle. Changing the excitation and emission property of the gold nanoparticles by making alloy nanoparticles of gold and other transition metal atoms such as silver, platinum or palladium could be possible because the affinity to the electrons are different by each atoms which will result the change in orbital energy levels. As a demonstration of the specific delivery application, folic acid has been conjugated on the surface using two different chemistries via PEG linker and further investigation will be performed with the cancer cells. Nucleus localization sequence (NLS) can be further attached to make the particles specifically delivered the nucleus of cancer cells as illustrated in Figure 1. Interestingly, upon the surface modification with folic acid via PEG linker, the emission color has been changed from 620 nm (red) to 460 nm (blue) without changing the excitation wavelength (Figure 2). It can also be explained as meta-stable state orbital of the gold nanoparticle is disrupted by the surface modification. Figure 2. Pictures and emission plot @ 280 nm excitation of gold nanoparticles before and after folic acid attachment showing the change of the emission Even though it is still in the on-going stage, we have successfully developed water soluble fluorescent gold nanoparticles and showed some of the possible modifications for bio-imaging applications. Further studies including the changing the emission-excitation properties of nanoparticles, conjugation of other targeting molecules, and folic acid-positive carcinoma incubation for the folic acid conjugated gold nanoparticles will be performed as future work of this project. [1] Zheng et al., Physical Review Letters, 2004, 93, 77402-1~4.