342g Metal Core-Shell Nanomagnets as a Carrier for Magnetically Tagged Drugs
Agile and controllable drug carriers are highly demanded to ensure quick action and avoid high concentrations in the body. According to recent findings, nanomaterials seem to meet exactly these requirements [3]. However, the toxic potential of nanomaterials is not yet fully investigated [4]. Therefore, the carrier particle should be either self-destroyed by dissolution or removed completely from the human body after application. Magnetic particles open an easy way to direct the drug to the site of action and to separate it from the body when it is not needed any longer [5]. However, most currently used oxide nanoparticles are only weakly magnetic.
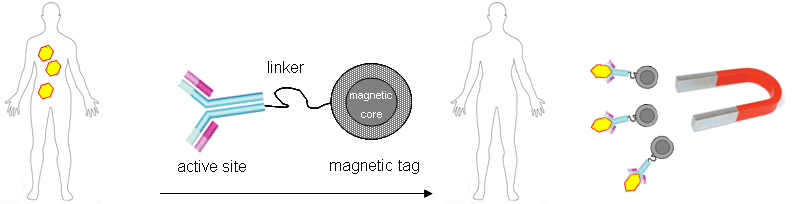
Recently, covalent surface functionalization of carbon-coated magnetic metal nanoparticles has proven to be successful and provide a much stronger magnetic carrier [6]. This provides a powerful linkage between the multi-facetted properties of magnetic core-shell nanoparticles and organic chemistry. Linker molecules connect the magnetic particles with e.g. proteins, such as therapeutic antibodies (Figure 1). The magnetically tagged antibody is applied like in a conventional therapy, but magnetic fields can guide the active substance to the desired target and finally remove the antibody-antigen complex efficiently.
[1] V. P. Torchilin, Europ. J. Pharm. Sci., 2000, 11(2), 81–91.
[2] L. J. Samoy, P. J. Zed, K. Wilbur, et al., Pharmacotherapy, 2006, 26(11), 1578-1586.
[3] K. K. Jain, Med. Princ. Pract., 2008,17, 89-101.
[4] L. K. Limbach, P. Wick, P. Manser, R. N. Grass, A. Bruinink, W. J. Stark, Environ. Sci. Technol., 2007, 41, 4084-4089.
[5] A. Ito, M. Shinkai, H. Honda, T. Kobayashi, J. Biosci. Bioeng., 2005, 100 (1), 1-11.
[6] R. N. Grass, E. K. Athanassiou, W. J. Stark, Angew. Chem. Int. Ed., 2007, 46, 4909-4912.