721d Separation of Chiral Amine by Capillary Electrophoresis
Stereochemistry can have a significant effect on the biological activity and the side effects of a drug, different from those of the optically pure drugs. Therefore, the development of a chiral separation method for the determination of optical purity is desirable in many areas.
In the more conventional chromatographic procedures such as high performance liquid chromatography (HPLC), gas chromatography (GC) and thin-layer chromatography, chiral separations were achieved by the use of chiral separations were achieved by the use of chiral additives in the mobile phase. Capillary electrophoresis provided a highly efficient separation method for chiral compounds by the use of a chiral selector in the running buffer. These chiral selectors used in chiral separation mainly include bile salts, chiral surfactants, chiral crown ethers, cyclodextrin derivatives and so on. In the chiral separation for capillary electrophoresis, ¦Â-CD has been used extensively as chiral selector.
2-HPA is a new drug to cure the Parkinson's disease and only the R-isomer is pharmacological activity [1]. In order to separate 2-HPA we have experienced some methods and found that separation of 2-HPA by capillary electrophoresis was efficient[2]. Further more the precursor 2-HA and outgrowth 2-HPPA were separated. Because there were not ultraviolet absorbing groups in 2-HPA and 2-HA, dansyl chloride was chosen to derivatize 2-HPA and 2-HA. 2-HPPA was separated directly.
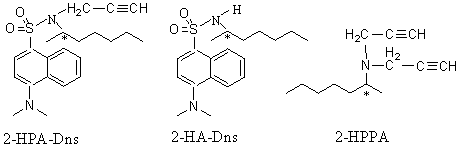
FIG.1 Structures of three chiral compounds
Experimental:
Reagents and Instrumentation: 2-HA (Adrich, 99%), PBS (Adrich, 96%), ¦Â-CD (Sigma, 99%), others are chemical pure. CE apparatus (BINDA), capillary columns (50¦Ìm¡Á70cm).
Derivation: 1ml 2-HPA, 1ml dansyl chloride (9g/l) and 15ml Na2CO3 aqueous solution (pH=11) were stirred for 15 minutes at 60¡æ in water bath, and then cooled to the ambient temperature. 15ml of ethyl acetate was used to extract the derivatized compounds. The extract was blew with the nitrogen under 40¡æ.
Chromatographic conditions: A suitable chiral analysis conditions in capillary electrophoresis was as following, 10mmol/l ¦Â-cyclodextrin dissolved in 20mmol/l PBS, pH=4.34; samples concentration 0.1g/l; temperature 25¡æ; separation voltage: 10kV; samples were injected into the capillary by 5kV for 5 seconds. The detection wavelengths were 210 nm and 254 nm. The capillary column was first flushed 10 minutes with the 0.1 mol/l NaOH, then with distilled water for 10 minutes, balanced with buffer liquid for about 10 minutes. In this experiment the samples and the buffer were degassed ultrasonically before use. Each component of the mobile phase was filtered through a 0. 45¦Ìm membrane.
Results and discussion
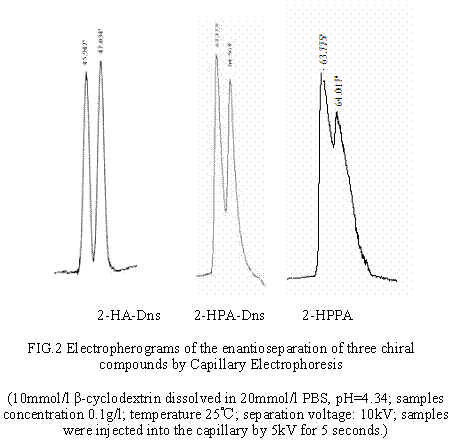
The result showed that pH of buffer played a key role in separation. The experiment proved that the speed of isomer moving toward to cathode decelerated under the low pH condition, so the coalescent time between ¦Â-cyclodextrin and isomer were prolonged. It was advantageous to separate. On the contrary, higher pH was disadvantageous in separation .We confirmed that the best separate pH condition was 4.34. The effect of chiral selector was important also. According to the Stephen's formula[3]: ¡安¦Ì={[C](¦Ì1-¦Ì2)(K2-K1)}/{1+[C](K1+K2)+K1K2[C]}, we choose the ¦Â-CD concentration 10mmol/l. Under the optimal condition 2-HPA-Dns was separated by based-line, 2-HA-Dns and 2-HPPA were separated well.
1. Effect of pH on ¡安¦Ì: We found that the pH was the significant factor affecting the chiral separation. And the ¡安¦Ì increased with a decrease in buffer pH. When the buffer pH was 4.34, ¡安¦Ì tended to a constant value. It is well known to us that the degree of protonation of enantiomers increases as the pH value of the buffer decreases, on the basis of the acid base equilibria of the R- and S-enantiomers. The effective mobilities are related to the degree of protonation of the enantiomers. The effective mobility of basic compounds increases with an increase in the hydrogen ion concentration of the buffer, which leads to an increase in ¡安¦Ì,. Additionally, the electrosmotic flow decreased with an increase in hydrogen ion concentration. The low pH of the buffer caused an improvement in the chiral separation.
2. Effect of ¦Â-CD concentration on ¡安¦Ì: The chiral recognition of ¦Â-CD is based on the difference in the formation constants of ¦Â-CD complexes with enantiomers, which reflect the difference in hydrophobic complex-forming interaction between the ¦Â-CD cavity and the guest molecule. The ¦Â-CD concentration is an important factor affecting the chiral separation. When the ¦Â-CD concentration was higher than 10 mmol/l, ¡安¦Ì tended towards a constant value.
3. Effect of electrolyte concentration on the chiral separation: The chiral separation of enantiomers improved with an increase in electrolyte concentration. This was because an increase in electrolyte concentration decreased the effects which were caused by the differences of the pH and electrolyte concentrations of the sample zone from that of the buffer. Moreover, the increase in electrolyte concentration made the electroomotic flow decrease, and led to an improvement in the resolution of enantiomers. However, the high concentration of electrolyte in buffer was not beneficial to chiral separation, due to a Joule heating effect.
Keyword: dansylchloride; chiral separation; capillary electrophoresis; 2-HPA
References
[1] David A£¬Durden, Lillian E. Drug Metabolism and Disposition, 28(2):147-154 (2000).
[2] Blomberg LG, Wang H., Electrophoresis, 21(10): 1940-1952 (2000).
[3] Wren SAC, Rowe R. C., J. Chromatogr, 603:235(1992).