496b Large-Scale Gas Phase Synthesis of Zinc Sulfide Nanoparticles
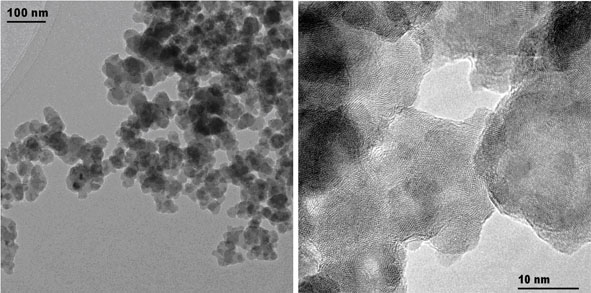
Here we present how thermodynamics allowed us to further extend the principle of flame synthesis for the production of sulfide nanoparticles. The oxygen-limiting conditions in collaboration with sufficient amounts of a sulfur source allowed the production of zinc sulfide nanoparticles (10 to 40 nm, Figure 1) at much higher production rates (in a lab-scale reactor: 5 to 10 g h-1) than typical wet-based processes of quantum dots. The zinc sulfide nanoparticles could be further doped with other elements to further enhance its electrical properties such as photoluminescence. We demonstrate how versatile gas phase processes are and how they could offer an interesting alternative for the large-scale production of photoluminescent materials.
Fig. 1: The principle of flame spray pyrolysis has been further extended for the preparation of zinc sulfide nanoparticles. Transmission electron microscopy images of zinc sulfide nanoparticles show that the as prepared particles have an average size of 10 to 40 nm (left) and exhibit high crystallinity (right).
[1] N. Osterwalder, S. Loher, R. N. Grass, T.J. Brunner, L. K. Limbach, S. C. Halim, W. J. Stark, J. Nanopart. Res., 2007, 9, 275.
[2] T.J. Brunner, M. Bohner, C. Dora, C. Gerber, W.J. Stark, J. Biomed. Mater. Res. Part B, 2007, DOI: 10.1002/jbm.b.30809
[3] E. A. Athanassiou, R. N. Grass, W. J. Stark, Nanotechnology, 2006, 17, 1668.
[4] R. N. Grass, W. J. Stark, J. Mater. Chem., 2006, 16, 1825.
[5] R.N. Grass, W.J. Stark, J. Nanopart. Res., 2006, 8(5), 729.
[6] R.N. Grass, M. Dietiker, C. Solenthaler, R. Spolenak, W.J. Stark, Nanotechnology, 2007, 18(3), 035703.
[7] R.N. Grass, E.K. Athanassiou, W.J. Stark, Angew. Chem. Int. Ed., 2007, in print
[8] E.K. Athanassiou, R.N. Grass, N. Osterwalder and W.J. Stark, Chem. Mater, 2007, 19, 4847.
[9] R.N. Grass, T.F. Albrecht, F. Krumeich, W.J. Stark, J. Mater. Chem., 2007, 17, 1485.