463a Direct Relationship Between Enhanced Gene and Matrix Protein Expression by Osteoblasts Exposed to Bioactive Glass Ions
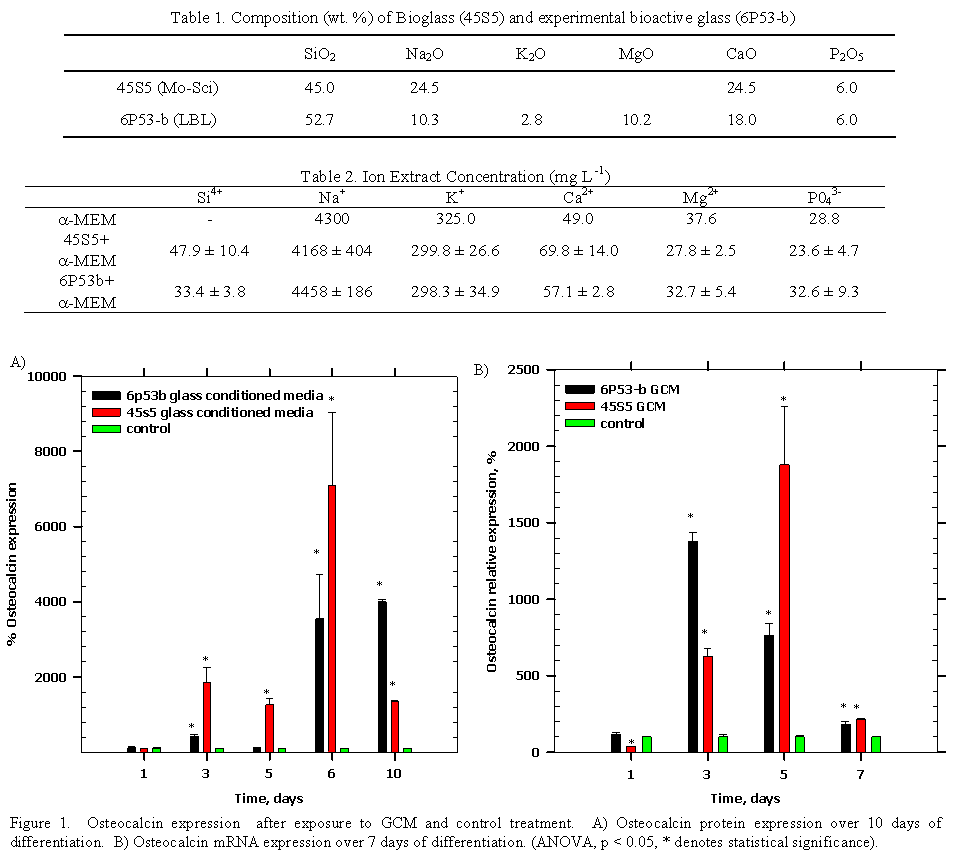
Introduction and Specific Aim¶ Currently, titanium (Ti) implants are used successfully for tooth replacements in mandibular or maxillary bone (86% and 76% success rate, respectively [1]) and for bone replacement in the cranium (nearly 93% success rate [2]). However, faster bone integration and improvements in implant success rate and longevity are still needed. A new family of bioactive glasses (50-59 wt. % SiO 2) may enhance the osteointegration potential of Ti [3-15]. The bioactive glasses acts as a functionally graded interface that remains well adherent to titanium [15] while also forming a hydroxyapatite surface layer and releasing calcium and silicon ions to osteoblasts for direct bone bonding [14, 16].¶ Interestingly, it has been discovered that these ions released to osteoblasts may play an active role in osteoblast function. Foppiano and colleagues [17] and Hench and colleagues [18-27] found these ions enhance osteoblast gene expression, yet, no significant effort on downstream matrix protein production has been established. Thus, this study tested the hypothesis that bioactive glass ions enhance osteogenesis and that a direct connection exists between enhanced gene expression and enhanced matrix protein synthesis. The key osteogenic markers to be investigated here are collagen type 1 alpha 1 (Col1α1) and alpha 2 (Col1α2), core binding factor a (Cbfa1/Runx2), alkaline phosphatase (ALP), and osteocalcin.¶ Materials and Methods¶ Melt-derived experimental bioactive glass (6P53-b) and commercial Bioglass (45S5) were used in this study by soaking glass specimens in cell culture medium to make glass conditioned medium (Table 1). The 6P53 b and 45S5 glass conditioned mediaum were measured for their ion concentrations using inductively coupled plasma mass spectrometry (ICP MS) prior to addition to mouse osteoblast progenitors (MC3T3 E1.4, at confluence in multi-well plates). The cells were induced to differentiate by adding ascorbic acid (50 μg mL-1) to GCM and control media. Cells were lysed for both mRNA and protein for sample analysis. Gene expression (Col1α1, Col1α2, Runx2, osteocalcin) was measured using quantitative reverse transcription-polymer chain reaction (qRT PCR) and amplification curve results were analyzed using a sigmoidal curve fitting method [28]. Protein expression (ALP, osteocalcin) was measured using enzyme-linked immunosorbent assay (ELISA). Statistical comparisons (p < 0.05 for statistical significance) were made between GCM- and control-treated cells using two-way ANOVA (independent variables: time, treatment; dependent variables: gene, protein expression) and Tukey's for individual group comparisons. Results It was observed that increased silicon and calcium concentrations were contained in 45S5 and 6P53-b GCM as compared to control media (α-MEM) (Table 2). GCM treatment enhanced osteocalcin expression (Figure 1). The expression of osteocalcin peaked (Figure 1A) for cells treated with ascorbic acid and 6P53 b GCM (40x control after 10 days) and 45S5 GCM (70x control after 6 days). These significantly increased osteocalcin protein levels correlated with the increased osteocalcin mRNA levels within 7 days of treatment. While MC3T3-E1.4 cells differentiated, osteocalcin gene expression reached a maximum after 5 days in 6P53 b GCM (14x control) and after 3 days in 45S5 GCM (19x control) (Figure 1B). Other markers measured included: Runx2 levels were approximately 2x control after 7 days exposure to GCM, which confirmed the results of Foppiano and colleagues [17]. Alkaline phosphatase activity was also 2x control for both GCM treated cells after 10 days of exposure. Finally, Col1α1 expression was enhanced after 1 day of exposure (45S5 GCM: 3x control; 6P53 b GCM: 4x control) and throughout the course of differentiation (day 5, Col1α2, 45S5 GCM: 3.15x control; 6P53 b GCM: 2.35x control).¶ Conclusions¶ This study tested the hypothesis that bioactive glass ions enhance osteogenesis and that there is a direct connection between enhanced gene expression and protein matrix production. The increased gene expression was seen for collagen type 1, Runx2, and osteocalcin, which fits into the known sequence of events of osteoblast differentiation in that collagen type 1 expression is followed by Runx2 expression and these two markers are necessary for downstream expression of osteocalcin and alkaline phosphatase [29]. These results are significant because to this point, no connection has been made to the enhanced gene expression by osteoblasts exposed to bioactive glass ions and their enhanced downstream matrix protein expression. Future research will explore the underlying extracellular and intracellular mechanisms that cause enhanced differentiation and impact mineralized-tissue formation.¶ References¶ [1] R. Adell, U. Lekholm, B. Rockler and P. I. Branemark, Int J Oral Maxillof 10 (6), 387-416 (1981).¶ [2] B. A. Miles, D. P. Sinn and G. G. Gion, J Craniofac Surg 17 (5), 889-897 (2006).¶ [3] E. Saiz, M. Goldman, J. M. Gomez-Vega, A. P. Tomsia, G. W. Marshall and S. J. Marshall, Biomaterials 23) 3749–3756 (2002).¶ [4] A. Pazo, E. Saiz and A. P. Tomsia, Acta Mater 46 (7), 2551-2558 (1998).¶ [5] T. Oku, K. Suganuma, L. R. Wallenberg, A. P. Tomsia, J. M. Gomez-Vega and E. Saiz, J Mater Sci-Mater M 12 (5), 413-417 (2001).¶ [6] S. Lopez-Esteban, E. Saiz, S. Fujino, T. Oku, K. Suganuma and A. P. Tomsia, J Eur Ceram Soc 23 (15), 2921-2930 (2003).¶ [7] J. M. Gomez-Vega, E. Saiz, A. P. Tomsia, T. Oku, K. Suganuma, G. W. Marshall and S. J. Marshall, Adv Mater 12 (12), 894-898 (2000).¶ [8] J. M. Gomez-Vega, E. Saiz, A. P. Tomsia, G. W. Marshall and S. J. Marshall, Biomaterials 21 (2), 105-111 (2000).¶ [9] J. M. Gomez-Vega, E. Saiz and A. P. Tomsia, Journal of Biomedical Materials Research 46 (4), 549-559 (1999).¶ [10] J. M. Gomez-Vega, A. Hozumi, E. Saiz, A. P. Tomsia, H. Sugimura and O. Takai, Journal of Biomedical Materials Research 56 (3), 382-389 (2001).¶ [11] S. Fujino, H. Tokunaga, E. Saiz and A. P. Tomsia, Mater Trans 45 (4), 1147-1151 (2004).¶ [12] S. Fujino, K. Morinaga, E. Saiz and A. P. Tomsia, Glass Sci Technol 75, 221-226 (2002).¶ [13] S. Foppiano, S. J. Marshall, E. Saiz, A. P. Tomsia and G. W. Marshall, Acta Biomaterialia 2:133-142 (2006).¶ [14] S. Foppiano, S. J. Marshall, G. W. Marshall, E. Saiz and A. P. Tomsia, J Biomed Mater Res A 71A (2), 242-249 (2004).¶ [15] D. R. Bloyer, J. M. Gomez-Vega, E. Saiz, J. M. McNaney, R. M. Cannon and A. P. Tomsia, Acta Mater 47 (15-16), 4221-4224 (1999).¶ [16] V. G. Varanasi, T. Vallortigara, P. M. Loomer, E. Saiz, A. P. Tomsia, S. J. Marshall and G. W. Marshall, presented at the Materials Research Society Symposium Proceedings, San Francisco, CA, 2006 (unpublished).¶ [17] S. Foppiano, S. J. Marshall, G. W. Marshall, E. Saiz and A. P. Tomsia, Acta Biomaterialia 3 (5), 765-771 (2007).¶ [18] I. D. Xynos, M. V. J. Hukkanen, L. L. Hench and J. M. Polak, J Pathol 187, 38a-38a (1999).¶ [19] I. D. Xynos, M. V. J. Hukkanen, J. J. Batten, L. D. Buttery, L. L. Hench and J. M. Polak, Calcified Tissue Int 67 (4), 321-329 (2000).¶ [20] I. D. Xynos, A. J. Edgar, M. Ramachandran, L. D. K. Buttery, L. L. Hench and J. M. Polak, J Pathol 193, 31a-31a (2001).¶ [21] I. D. Xynos, A. J. Edgar, L. D. K. Buttery, L. L. Hench and J. M. Polak, Journal of Biomedical Materials Research 55 (2), 151-157 (2001).¶ [22] I. D. Xynos, A. J. Edgar, L. D. K. Buttery, L. L. Hench and J. M. Polak, Biochem Bioph Res Co 276 (2), 461-465 (2000).¶ [23] J. R. Jones, P. Sepulveda and L. L. Hench, Journal of Biomedical Materials Research 58 (6), 720-726 (2001).¶ [24] J. R. Jones and L. L. Hench, Curr Opin Solid St M 7 (4-5), 301-307 (2003).¶ [25] L. L. Hench and J. M. Polak, Science 295 (5557), 1014 (2002).¶ [26] R. C. Bielby, R. S. Pryce, L. L. Hench and J. M. Polak, Tissue Eng 11 (3-4), 479-488 (2005).¶ [27] R. C. Bielby, I. S. Christodoulou, R. S. Pryce, W. J. P. Radford, L. L. Hench and J. M. Polak, Tissue Eng 10 (7-8), 1018-1026 (2004).¶ [28] H. Qiu, K. Durand, H. Rabinovitch-Chable, M. Rigaud, V. Gazaille, P. Clavere and F. G. Sturtz, Biotechniques 42 (3), 355-362 (2007).¶ [29] G. Z. Xiao, R. Gopalakrishnan, D. Jiang, E. Reith, M. D. Benson and R. T. Franceschi, J Bone Miner Res 17 (1), 101-110 (2002).¶