558b Low Temperature Small Scale Reaction Calorimetry with in-Situ ATR Spectroscopy for Tracking Lithiation and Electrophilic Addition in Alkylation/acylation Reactions
Fine chemical processes are still often optimized with off-line methods, i.e. for varying operating conditions product(s) and/or stable intermediate(s) are analyzed in order to determine the most appropriate process conditions. However, online or in-situ analytical methods such as time resolved calorimetry and spectroscopy supply significantly more information. This can encompass simple hints to the existence and structure of metastable intermediates and mechanistic steps that ultimately lead to a detailed kinetic and mechanistic model. This allows a more precise prediction of optimal reaction conditions that can even be outside the investigated operating range, an important aspect also for up-scaling [1].
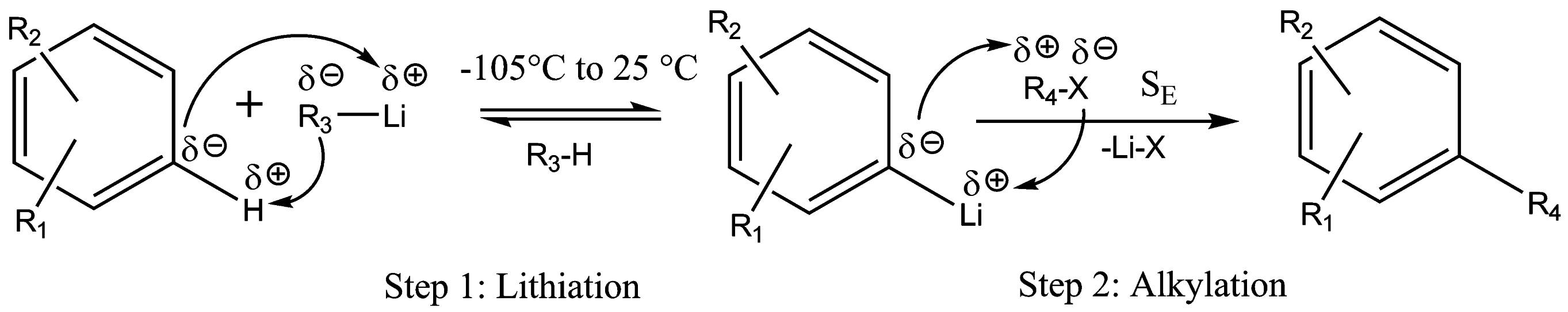
In fine chemical industry, selective alkylation/acylation is one of the most fundamental and important classes of reactions. Amongst other methods, the use of organolithium compounds is a modern and powerful technique for C-C bond formation. Lithiation reactions are generally catalyzed by solvent(s), e.g. TMEDA, and performed at low temperature ranging from as low as -105°C up to 25°C [2].
For the past decade, we have been developing small scale reaction calorimeters (25-50mL) based on the principles of power compensation and heat balance, employing reactor vessels surrounded by a metal jacket that is thermostated by PID controlled Peltier elements [3,4]. In its latest generation, we present an extended low temperature version allowing for time resolved calorimetric investigations with in-situ mid-IR and/or UV-Vis ATR spectroscopy down to -70°C.
In industry, it is of great importance to optimally schedule the dosing of the alkylation/acylation electrophile (Step 2 in the Figure) to avoid recombination with the nucleophilic lithiation agent (e.g. n-BuLi) remaining from Step 1. For a case study, the small scale reaction calorimeter with its hyphenated analytical devices is applied to tackle this task and help optimizing other reaction conditions such as initial concentrations and reaction temperature. Well established mathematical soft- and hard-modeling methods [5,6] are used to analyze the experimental data. Ultimately, this can help us to find a mechanistic model with its intrinsic kinetic and thermodynamic parameters.
1. Rubin A.E., Tummala S., Both D.A., Wang C., Delany, E.J. Emerging Technologies Supporting Chemical Process R&D and Their Increasing Impact on Productivity in the Pharmaceutical Industry. Chemical Reviews (2006) 106: 2794-2810.
2. Rappoport Z. and Marek I. The chemistry of organolithium compounds. Wiley, Chichester 2004.
3. Zogg A., Fischer U., Hungerbühler K. A New Small Scale Reaction Calorimeter That Combines the Principles of Power Compensation and Heat Balance. Industrial and Engineering Chemistry Research (2003) 42: 767-776.
4. Visentin F., Gianoli S.I., Zogg A., Kut O.M., Hungerbühler K. A pressure-resistant small-scale reaction calorimeter that combines the principles of power compensation and heat balance (CRC.v4). Organic Process Research & Development (2004) 8: 725-737.
5. Puxty G., Fischer U., Jecklin M., Hungerbühler K. Data-oriented process development: determination of reaction parameters by small-scale calorimetry with in situ spectrospcopy. Chimia (2006) 60: 605–610
6. Maeder M. and Neuhold Y.M. Practical Data Analysis in Chemistry. Elsevier, Amsterdam 2007.