314b Reaction Path Analysis of the Total Oxidation of Propane Over a Cuo-CeO2/γ-Al2O3 Catalyst by Means of Temporal Analysis of Products (TAP)
Abstract
Total oxidation of volatile organic compounds using metal oxide catalysts has become a necessary end-of-pipe process in order to reduce VOCs emissions. A TAP reactor is applied to investigate the mechanism and kinetics of propane oxidation over a CuO-CeO2/γ-Al2O3 catalyst. Both single-pulse and multi-pulse experiments are performed between 523 and 923 K using various feeds: pure C3H8, O2 and CO2 next to mixtures C3H8/O2 and C3H8/CO2. At 523 and 573 K propane interacts irreversibly with the oxidized catalyst, while no interaction with dioxygen is observed. From 623 K on both reactant responses show two maxima, indicating reversible interaction with the catalyst. At all temperatures, CO2 is the main reaction product both in the absence and presence of O2.
1. Introduction
Given the ever growing environmental concerns, volatile organic compounds (VOCs) should be eliminated as much as possible before releasing waste gases into the atmosphere. According to Spivey [1], catalytic oxidation is one of the most effective technologies for the destruction of VOCs. Supported noble metal catalysts have been established as efficient catalysts for this destruction. However, metal oxide catalysts appear to be a good economic alternative, in particular CuO-CeO2/γ-Al2O3 [2].
In this research, the total oxidation of propane as model molecule for VOCs is studied over a CuO-CeO2/γ-Al2O3 catalyst using the TAP reactor [3] to gain insight into the details of the reaction mechanism.
2. Experimental
The total oxidation of propane was studied, performing single-pulse and multi-pulse experiments on 50 mg of a CuO-CeO2/γ-Al2O3 catalyst. This amount of catalyst corresponds to 6.61 1019 O atoms based on both CuO and CeO2 present in the catalyst. This amount is assumed to be an upper limit for the total number of exchangeable O atoms. The experiments are carried out with different feeds: C3H8/Kr (90/10), O2/Ar (50/50), CO2/Ar (50/50), C3H8/Kr + O2 (O2/C3H8 ratio of 5) and C3H8/Kr + CO2 (CO2/C3H8 ratio of 5) and with varying temperatures in steps of 50 K between 523 and 923 K. The fresh catalyst samples are first heated to reaction temperature with a ramp of 5 K/min under vacuum. Subsequently, the catalyst is pretreated with multi-pulses of O2 until a constant level of the oxygen response is obtained.
3. Results and discussion
The analysis of the propane and dioxygen pulse responses at 523 and 573 K demonstrates that propane is irreversibly interacting with the oxidized catalyst, while dioxygen does not interact. As temperature is increasing, the responses start to show two local maxima. The first corresponds to a fast adsorption while the second is caused by slow desorption.
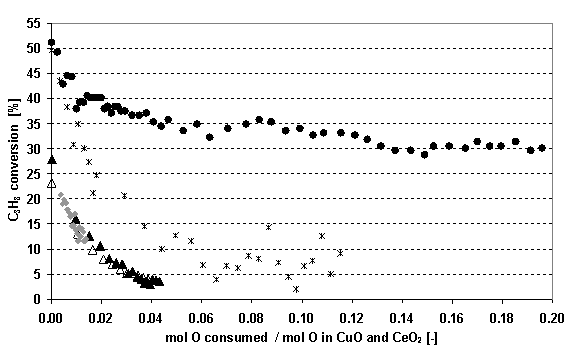
CO2 is the most important product at all temperatures during C3H8 oxidation, while only very small amounts of C3H6 are detected. Neither H2O, nor CO can be observed during single-pulse experiments, although H2O is sporadically detected on a scan, indicating that desorption takes place. The same products are present when a C3H8/O2 mixture is fed into the reactor, but in this case higher propane conversions are obtained. Figure 1 shows the propane conversion over 50 mg of catalyst at 623 K for three different feeds as a function of the ratio between the O consumption by reaction and the O initially present in the oxidized catalyst as CuO and CeO2. When pure propane is pulsed, the conversion is lower and decreases rapidly from 25% to around 3%. This indicates that reduction of the catalyst is taking place by using O atoms from the catalyst to form the oxidation products. Feeding dioxygen together with propane in a stoichiometric ratio of 5 to 1 causes a much less pronounced decrease in the conversion levelling off towards a value of 30%. This observation indicates that the dioxygen present in the feed is consumed by the catalyst in order to re-oxidize the sites that were reduced due to propane. However, since the average O2 conversion is only 10%, not all sites that were reduced during propane oxidation are re-oxidized by gaseous O2, leading to a decrease in the catalyst activity.
Figure 1: Comparison of the propane conversion at 623 K as a function of the oxygen consumption during reaction for three different feeds. When pure C3H8 is fed, the activity of the fresh catalyst is compared with the activity of the same catalyst sample that was subjected to reduction-oxidation experiments for 5 and 7 days: ♦ pure C3H8-fresh catalyst, ▲ pure C3H8-catalyst after use for 5 days, Δ pure C3H8-catalyst after use for 7 days, ● C3H8/O2 (ratio 1:5) and * C3H8/CO2 (ratio 1:5) The most surprising observation is that between 523 and 723 K the CO2 yield remains very low even if three times more catalyst is present in the reactor. Moreover, at these lower temperatures the CO2 yield does not increase when a C3H8/O2 mixture is fed instead of pure propane although the conversion is higher in the first case. Based on these observations, it is expected that part of the CO2 formed during reaction remains adsorbed on the catalyst surface. In order to investigate this issue into more detail, CO2 mixed with Ar is sent over the oxidized catalyst at different temperatures. At the lower temperatures (523-723 K), CO2 is strongly interacting with the catalyst since only a small amount is detected at the outlet. From 773 K on, considerably more CO2 is reaching the reactor outlet until at 923 K almost no CO2 adsorption is observed anymore. Because CO2 is staying adsorbed on the catalyst during reaction with C3H8 or C3H8/O2 at low temperatures, a possible change in activity can be expected if a catalyst sample is used in the TAP for a longer period of time. However, the catalyst activity is not influenced by this CO2 adsorption as is illustrated on Figure 1 (triangles), where the propane conversion at 623 K is presented at different times after a fresh catalyst sample was loaded into the reactor. Even if experiments involving reduction or total oxidation and subsequent re-oxidation are performed for 7 days on the same catalyst sample, no significant difference in propane conversion can be observed. When CO2 is added to the pure propane feed, the conversion at 623 K becomes significantly higher compared to when pure propane is fed into the reactor and initially reaches a level comparable to the conversion with a stoichiometric C3H8/O2 mixture (see Figure 1). 4. Conclusions The CuO-CeO2/γ-Al2O3 catalyst performs well as a total oxidation catalyst since no CO and almost no C3H6 is detected during reaction with C3H8 or C3H8/O2 between 523 K and 923 K. However, the catalyst interacts very strongly with CO2, leading to unclosed carbon balances at lower temperatures. This irreversible CO2 adsorption on the catalyst at low temperatures does not affect the activity. Acknowledgements This work was performed in the framework of a Concerted Research Action (GOA) financed by the References [1] J.J. Spivey, Ind. Chem. Res. 26, 1987, 2165-2180 [2] C.R. Jung, J. Han, S.W. [3] J.T. Gleaves, J.R. Ebner, T.C. Kuechler, Catal. Rev. Sci.