272b Antibacterial Silver-Containing Nanocomposites for Bone-Defect Repair
In vitro bioactivity tests of these wool-like electrospuncomposite PLGA/ATCP (80 : 20) containing 0.5 wt% silver showed rapid hydroxyapatite deposition on the nanocomposite within 2 days. Antibacterial tests using E. coli demonstrated a strongly prolonged antibacterial effect of the scaffolds containing finely dispersed silver on TCP if compared to current clinically used methods based on soaking the scaffolds with a tetracycline solution prior to implantation. These results indicate the potential for a broader use of silver in flexible implant materials and suggest applications in non-load bearing bone defects of lesions with high exposure to microorganisms, for example in dental surgery.
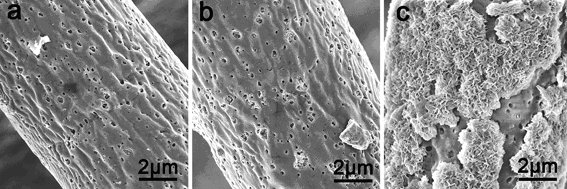
Figure: Scanning electron microscopy images after incubation in suspension of E. coli at 37 °C under shaking conditions: (a) PLGA/TCP 80 : 20 and (b) silver doped nanocomposite after 8 h immersion both showed small holes on the fibres; (c) silver containing nanocomposite after 160 h immersion (E. coli containing simulated body fluid changed almost every 12 h) showing the deposition of a hydroxyapatite layer.
References
[1] S. Loher, W.J. Stark, M. Maciejewski et al., Chem Mater 2005;17(1):36-42.
[2] O.D. Schneider, S. Loher, T.J. Brunner, L. Uebersax, M. Simonet, R.N. Grass, H.P. Merkle, W.J. Stark, J. Biomed Mater Res B, 2007, 84B(2), 350-62.
[3] O.D. Schneider, S. Loher, T.J. Brunner, P. Schmidlin, W.J. Stark, J. Mater. Chem., (2008)