222e Investigation of the Effect of Spillover Processes on the Catalytic Activity Using Mixed Ionic Electronic Conducting Membrane Reactors
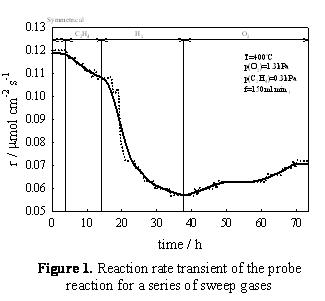
The modification of catalytic activity due to spillover processes between the catalyst and the support has been the subject of investigation by several research groups [1]. The differing activities of the catalyst and support create intra-catalyst chemical potential differences that allow these spillover processes to occur. By using a membrane analogue to a real catalyst we are able to demonstrate the effect of chemical potential differences to the catalytic activity. A novel configuration using a mixed ionic-electronic conducting (MIEC) support has been developed for this work [2]. The mixed conductivity of the support allows the supply of the promoter to the reaction catalyst by introducing chemical potential differences across the membrane via the use of appropriate sweep gases. Initial results on a dual chamber pellet membrane reaction proved the feasibility of this concept [3]. Experiments were performed on a dual chamber membrane reactor. Further experiments were conducted on a dual chamber hollow fibre membrane reactor. In both cases on of the reactor chambers was used as the reaction chamber and one as the sweep chamber (where the supply of the promoter originates from). The MIEC membrane used was La0.6Sr0.4Co0.2Fe0.8O3-ä (LSCF) with a platinum catalyst deposited onto it. The supply of the promoter was ensured by the use of an oxygen sweep gas, while hydrogen and ethylene were used for the removal of the promoter and reverse of the rate modification. Ethylene oxidation was the probe reaction Figure 1 shows the reaction rate transient obtained for a series of different sweep gases. We can see that the initial rate can be decreased by 50% by the combined use of an ethylene and a hydrogen sweep, while the use of oxygen as the sweep gas visibly increases the reaction rate. The phenomenon observed here is not fully reversible. Work is in progress to identify the mechanisms that govern the rate modification and explain the type and magnitude of the effect of each sweep gas. A different configuration featuring a hollow fibre membrane reactor is currently being investigated. The higher surface areas (both membrane and catalyst) of this reactor are expected to play an important part in the magnitude of the observed rate modification. A membrane-supported catalyst was used in order to investigate the effect of spillover on the catalytic activity. Experimental results demonstrated controllable rate modification as a result of chemical potential differences across the catalyst-system. References 1. B. Delmon, Catalysis Today, 2006, 117, 69-74. 2. D. Poulidi, A. Thusrfield, I.S. Metcalfe, Topics in Catalysis, 44 (2007), 435. 3. D. Poulidi, C. Anderson and I.S. Metcalfe, Solid State Ionics, In press, doi:10.1016/j.ssi.2008.01.056