265a Catalytic Effects of Minerals Matters on Stable Surface Oxides and Oxidation Rate In the Early Stage of Coal Combustion
Reactivity of old chars has been characterized by temperature-programmed desorption (TPD) of surface oxides up to 1000 °C in the past. In a recent study, we discovered the existence of stable surface oxides on young chars that desorb only above 1100 °C.1 After the wall interferences are minimized, we observed that oxygen from gas phase, organic portion of the coal and minerals in the coal have profoundly influences on the formation of stable surface oxides in the early stage of coal combustion.2 In an attempt to isolate the effects of minerals, demineralized coals (DMC) are oxidized in O2 with a contact time less than 1 second, and the amount and strength of stable surface oxides are characterized by TPD up to 1650 °C. Young chars derived from both demineralized lignite and bituminous coal show a low and flat TPD profiles over a wide temperature range signifying the minerals' catalytic activities in forming stable surface oxides, possibly in the adsorption step, for both coals. Indeed, the rates of oxidation of chars from both bituminous coal and lignite based on CO2 and CO yields during oxidation show higher rates than their DMC counterparts. Moreover, the rate of oxidation seems to be qualitatively correlated to the amount of surface oxides. Similar to those from raw coals, combined oxygen balance and elemental analysis of chars from DMC suggests that that oxygen in the organic portion of the lignite activates oxygen turnover and carbon oxidation during its combustion; chars from neither raw nor demineralized bituminous coal possesses these properties. Minerals are known to play a significant catalytic role for combustion of lignite; it appears that after removing the minerals from bituminous coal both reaction rate and surface oxides released during TPD decrease too. XPS spectra of raw and demineralized bituminous coal and their char show peaks around 532.0 eV in O(1s) difference spectrum suggesting the existence of stable surface oxides.
Reference
1. Chen, W.Y., Wan, S., Shi, S., “Stable Surface Oxides on Chars and Impact of Reactor Materials at High Temperatures,” Energy & Fuels, 2007, 21, 778-792.
2. Chen, W.Y., Shi, G., Wan, S., “Stable Surface Oxides on Coal-Derived Chars,” to be submitted to Energy & Fuels, 2008.
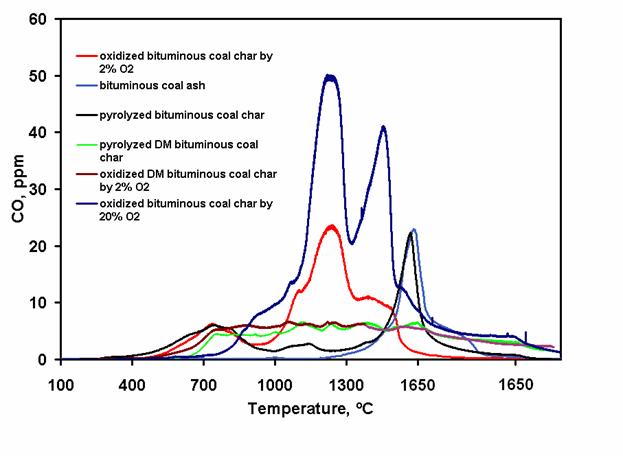
Figure 1. Temperature-program desoption (TPD) profiles of char oxidized by 2% O2 and with 15% carbon burnout at 900 °C, char oxidized by 21% O2 and with 75% carbon burnout, ash, pyrolyzed char, demineralized char oxidized by 2% O2 and with 15% carbon burnout, and pyrolyzed demineralized char (all normalized based on 1 mg carbon in char). Peaks of the oxidized chars at 1250 °C suggest the existence of stable surface oxides that controls the rate of char oxidation in flame conditions. Peaks at higher temperatures (1450 and 1550 °C) represent interactions between the SiC tube and minerals in the coal. Chars derived from oxidation of DMC (lignite and bituminous) show low and flat TPD profiles. Moreover, DMC's also have lower oxidation rates. These observations suggest the possible catalytic role of minerals in the early stage oxidation of both lignite and bituminous coal. Keywords: oxides, demineralized coal, oxidation, young char * for presentation at the annual meeting of the American Institute of Chemical Engineers, Philadelphia, PA, November 16-21, 2008. Wei-Yin Chen, contact author, Tel.: (662) 915-5651, Fax: (662) 915-7023, E-mail: cmchengs@olemiss.edu