569h Micro Thermal Ablation for Transdermal Drug Delivery
Introduction
The traditional passive transdermal patch systems have been limited to only small molecular weight and moderately lipophilic drugs due to the barrier properties of the skin, which reside in the cornified layer of keratinized epidermal cell, stratum corneum. Many research groups have demonstrated that the removal of stratum corneum could enhance the drug permeability to the skin and designed a number of active transdermal applications to overcome the stratum corneum barrier and deliver drugs efficiently in painless ways.
Although the main function of the skin is the protection of the human body from the external environment, it is composed of mechanically weak biomaterials. Thus, sufficient energy to ablate the skin can be applied to the skin. This has been demonstrated using lasers, RF heating using microelectrode arrays, and conductive heating from ohmic microheaters. Phase 1 and 2 clinical trials are underway using the latter two methods to deliver various drugs into the skin.
The key to success with thermal ablation of skin is to apply extremely short thermal pulses that heat just the surface of the skin, and thereby remove microscopic patches of the rate-limiting stratum corneum layer on the skin surface without damaging deeper, viable tissue. Because heating occurs on the skin surface, the short pulse assures a steep temperature gradient that does not persist long enough for significant thermal diffusion to deeper tissue. Typical ablation temperatures are hundreds of degrees Celsius and stratum corneum thickness is just 10 – 20 microns. Thermal pulses applied using current technology are milliseconds long.
In this study, we sought to apply microsecond thermal pulses of hundreds of degrees Celsius to ablated skin's stratum corneum. For the first time, we developed an arc-based energy release to achieve the extremely rapid heating.
Methods and Materials
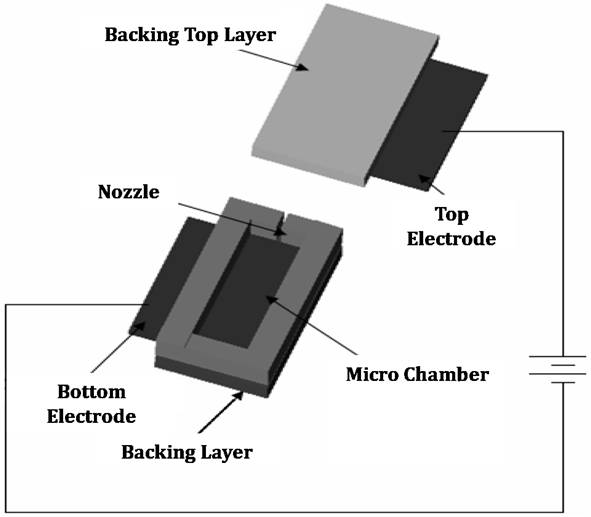
A microdevice shown in Figure 1 was designed and fabricated to generate the ablation energy. The system has a 1 µl microchamber having two metal electrodes on each side and a nozzle at top; and a power supply circuit with discharging capacitors providing the electrical energy to the electrodes. The microchamber was filled with PBS serving as the medium through which the arc discharge occurs. The metal mask covered the outer surface of the nozzle and additional masks were used to localize the skin ablation effect.
The ablation energy generated by the system was simulated with the software COMSOL MULTIPHYSICSбу and the temperature was measured by using thermal indicating papers coated with heat sensitive polymer film, which turns transparent and shows the color of the background. The thermal paper was placed on the metal mask exposed to hot medium ejected from the system and the temperature of hot medium was computed on the basis of the temperature measurement. The recoil force generated by arc discharge was measured by the force sensor installed at the back side of the microchamber. The force sensor recorded the change of the generated recoil force according to the time after triggering arc discharge phenomenon in the microchamber.
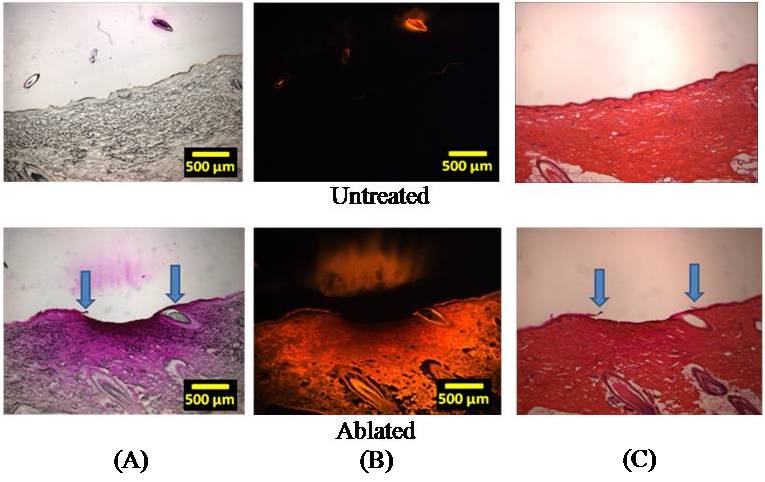
For histological analysis, shaved pig cadaver skin was placed on the metal mask in the same way as when measuring the temperature. Then, a viscous drug solution having a model drug, sulforhodamine, was mounted on the ablated skin surface to visualize the effect of the skin ablation on drug permeation. Tissue samples were imaged by brightfield and fluorescent microscopy, cryo-sectioned, and stained with hematoxylin and eosin to observe the removal of stratum corneum.
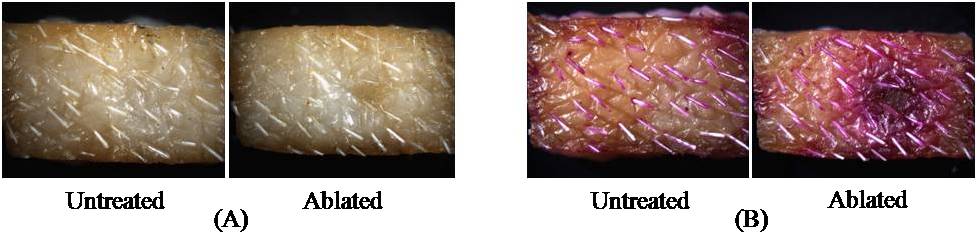
Figure 1.Schematic of micro thermal ablation system. Results and Discussion In this study, the ablation system was operated using a voltage of 100-150 V, which was experimentally determined as the threshold voltage range to obtain reliable skin ablation. The amount of voltage required to cause an arc discharge is strongly dependent on the gap between electrodes. The total energy stored in the system while supplying electrical energy was simulated between 4 and 5 J (data not shown) and the temperature of water medium filled in the microchamber was computed up to 600oC with the assumption that the energy generated by arc discharge phenomenon between two electrodes was used to heat the total amount of medium in the microchamber (calculation not shown). Compared to the other ablation techniques using the energy range of 30-50 mJ [1,2], the amount of energy released from this system is higher almost by 2 orders of magnitude. Because direct measurement of the temperature of the ejected medium was not easy due to the high speed arcing within the microsecond timescale, we measured the temperature of heat energy transferred through the metal mask from the ejected hot medium. The temperature of heat energy on the underside of a 25 µm-thick tungsten mask was measured to be at least 290oC. To determine the temperature of the jet that impacts the top side of the mask, we carried out a simulation, which predicted the temperature of the jet to be at least 1000oC. The recoil force generated by the system ranged from 1 to 2.5 N over a timescale of less than 100 µs. This force may be sufficient to have mechanical effects on the skin. Our research is continuing to address the relative roles of the high temperature and the high force of the arc-generated jet on the skin. Guided by this physical characterization of the arc-based jet, we next examined its effect on the skin. After ablating the skin in this way, the selective removal of stratum corneum was achieved. Figure 2A shows the surface of the untreated skin (control) and the ablated skin after applying the system to the skin surface and Figure 2B shows the same sample after 12 h delivery of the model drug, sulforhodamine. Comparing to the control sample, the skin surface around the ablated site showed the radially decreasing intensity of purple staining by sulforhodamine, implying the diffusion of sulforhodamine into the skin. This diffusion was identified with histological examination shown in Figure 3. Figure 2. The surface of pig cadaver skin before (A) and after (B) delivering sulforhodamine for 12 h. While the control sample shows no diffusion of hydrophilic sulforhodamine, the brightfield and fluorescent images (Figure 3A and 3B) of the ablated skin sample show a gradient in sulforhodamine from the ablated top to the deeper tissue and fluorescence due to the diffused sulforhodamine, respectively. Figure 3C shows a cross section of the stained skin sample which had intact stratum corneum and which lost stratum corneum without epidermis damage. Furthermore, an additional mask having the localizing windows enabled the separated and controlled effect of the heat ablation. It is highly probable that the localization of the skin ablation can determine the release rate of drug by controlling the area where the diffusion occurs. Figure 3. Histological images of untreated (above) and ablated (below) skin samples. Brightfield (A), Fluorescent (B), and H&E Stained (C). We expect that the selective removal of stratum corneum in this way can enhance the skin permeability of high molecular weight and hydrophilic biomolecule drugs. Moreover, the skin ablation using this system was completed within the microsecond timescale so that this system might be an effective and safe transdermal delivery technique based on heat ablation. Conclusion A micro thermal ablation system based on arcing was designed to generate and deliver the heat ablation energy to the skin on the microsecond timescale, which is faster than previous millisecond systems. Arc discharge phenomenon released the high heat energy sufficient to ablate the skin and thereby selectively remove stratum corneum. This was enabled by the microsecond timescale of ablation minimized thermal diffusion to deeper tissue. Through the micro size channel created by the heat ablation, hydrophilic molecule, sulforhodamine, was effectively delivered into the skin, which might enable the transdermal delivery of other hydrophilic drugs, including macromolecules. Reference 1. Steven L. Jacques, Daniel J. McAuliffe, Irvin H. Blank, John A. Parrish (1987), "Controlled Removal of Human Stratum Corneum" The journal of investigative dermatology, Vol. 88, pp. 88-93. 2. Basil M. Hantash, Vikramaditya P. Bedi, Bhumika Kapadia, Zakia Rahman, Kerrie Jiang, Heather Tanner, Kin Foong Chan, Christopher B. Zachary (2007), "In Vivo Histological Evaluation of a Novel Ablative Fractional Resurfacing Device" Lasers in Surgery and Medicine, Vol.39, pp.96-107