575x Performance of Au-Mg/Al2O3 Catalyst for the Selective CO Oxidation In Hydrogen Rich Stream
- Introduction
The fuel cell is considered to be one of the most attractive, pollution free energy conversion technologies for the future. Due to the technical difficulties in the safe storage of hydrogen, its on-board production using a fuel processor seems to be the most feasible alternatives for the utilization of fuel cells in transportation vehicles and medium size stationary applications in the near future [1-2]. However, CO in the hydrogen stream from the fuel processor is harmful for the anode catalyst of the fuel cell, and it should be eliminated using a suitable method. The supported noble metal catalysts (especially Pt) were studied extensively for this purpose [3]. Promotion of noble metal catalysts with a metal oxide also seems to improve the catalytic performance [3-5].
Gold was not considered as an effective catalyst until 1987, when it was reported that some gold catalysts prepared by co-precipitation method were extraordinarily active for the low temperature CO oxidation [6]. Then numerous works on the CO oxidation on the gold catalysts in the absence and presence of hydrogen has been reported [7-10]. The nature of the support or promoter (usually reducible metal oxides) also seems to play a significant role.
In this work, the CO oxidation over Au-Mg/Al2O3 catalyst was studied. The effects of catalyst preparation conditions as well as the reaction parameters such as temperature, CO2 and H2O content in the feed, and time on stream were investigated.
- Experimental
The composite MgO/Al2O3 supports were prepared via incipient wetness impregnation [7]. 1 wt.% Au was prepared by homogenous deposition precipitation (HDP) using urea as a precipitation agent. An aqueous solution of HAuCl4.3H2O and urea was added to MgO/Al2O3 with vigorous stirring, heated to 70 oC and kept at this temperature until the pH reached to 7-8. Then the suspension was cooled down, vacuum filtered and washed with de-ionized water and dried at 100 oC. Prior to reaction, the catalysts were reduced in H2 environment and cooled down in He [8-10].
Temperature programmed reaction technique was used to investigate the activity of catalyst in the temperature range of 80-150 oC. The activity measurements were performed in a micro-reactor flow system with the feed (100 ml/min) composition of CO = 1.0%, O2 = 1.0 %, H2 =60 %, He = 3%, CO2 =25 %, H2O= 10 % over 250 mg of catalysts. Product streams were analyzed by HPR-20 mass spectrometer for the calculation of CO conversion.
3. Results and Discussion
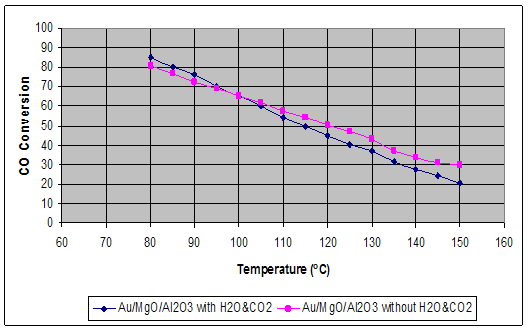
The activity and stability of Au-MgO/Al2O3 catalyst in the preferential CO oxidation in hydrogen rich stream were investigated in the absence and presence of H2O and CO2. It was found that the presence of 25% CO2 and 10% H2O had positive effects at low temperature compare to absence of both while this effect was reversed at higher temperatures than 100 oC (Figure 1.)
Figure 1. The effects of temperature on CO conversion in the absence and presence of CO2 and H2O Then the effects of CO2 and H2O were investigated in details. It was found that the presence of 5% CO2 in the feed decreased the CO conversion about 10% while the further increase did not change this effect much. The addition of 10% H2O, on the other hand, balanced the negative effects of CO2 significantly although 5% H2O did not make much difference. The effect of Mg loading was also studied, and it was observed that increasing Mg loading form 1.25 wt % to 2.5 wt % improved CO conversion at high temperatures while this effect diminished below 100 oC, and the further increase of Mg to 5% decreased CO conversion significantly. Finally the stability test for 1 wt% Au-1.25% Mg /Al2O3 catalyst was performed and no activity lost was observed for about 20 hours. Acknowledgement This work was supported by The Scientific and Technical Research Council of Turkey through project 105M034 and Bogazici University Research Fund through project 06M104 References: 1. T. R. Ralph, G. A. Hards,