510f Investigations of Transient and Equilibrium Zeta Potential on Mica and Silica
The advent of a relatively new technique for measurement of zeta potential, the rotating disk, has made the investigation of the interaction between a substrate and solution in the first moments after immersion. The substrate is suspended in air above the solution; rotation at 2000 rpm is begun; a nanovoltmeter is actuated to begin recording the potential difference between sensors already in solution; and the substrate is quickly immersed and placed into position for recording streaming potential.
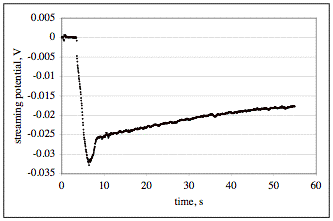
The figure: The graph shows streaming potential data acquired from two sensors in the same cell with a 25 mm diameter disk of mica rotating at 2000 rpm in distilled water. One sensor (Ag/AgCl reference electrode) is placed on the axis of rotation 1 mm from the sample and the other sensor is farther away. The initial measurement of potential difference is zero because the disk is spinning above the air/solution interface. The measurement decreases instantly as the sample is immersed at around 4 seconds after turning on the data acquisition; this decrease accurately marks the zero of time of immersion. As the sample is lowered toward the axial sensor, the measured streaming potential changes by virtue of the motion. The sample is lowered to a position very close to the sensor and the retracted to the desired 1 mm separation from the sensor. This positioning causes the undershoot of the potential in the figure. Once the sensor is in position and not moving, meaningful measurements of a transient in the zeta potential of the mica were acquired. In this case, the streaming potential changes by at least 7 mV in the first minute. The streaming potential measurements are converted to zeta potential measurements by the theory of the streaming potential in the vicinity of a rotating disk.
A transient model for adsorption and desorption reactions occurring after immersion also has been developed in order to take advantage of this capability. The model expresses the rates of reactions between sites on the surface and charged solute species at the outer Helmholtz plane. The method and the model are described in a related presentation. While the focus of the earlier presentation was the derivation of the model and its verification, the focus of this presentation is on the results obtained for the interaction of mica and silica with alkali metal cations, protons, and more complex adsorbates such as amino acids. The relation between the findings of this new approach to probing the interactions of adsorbates with solids and prior equilibrium data and models is described.
Web Page: www.zetaspin.com