263a Bioactive Microgels for Large-Scale Eukaryotic Cell Culture
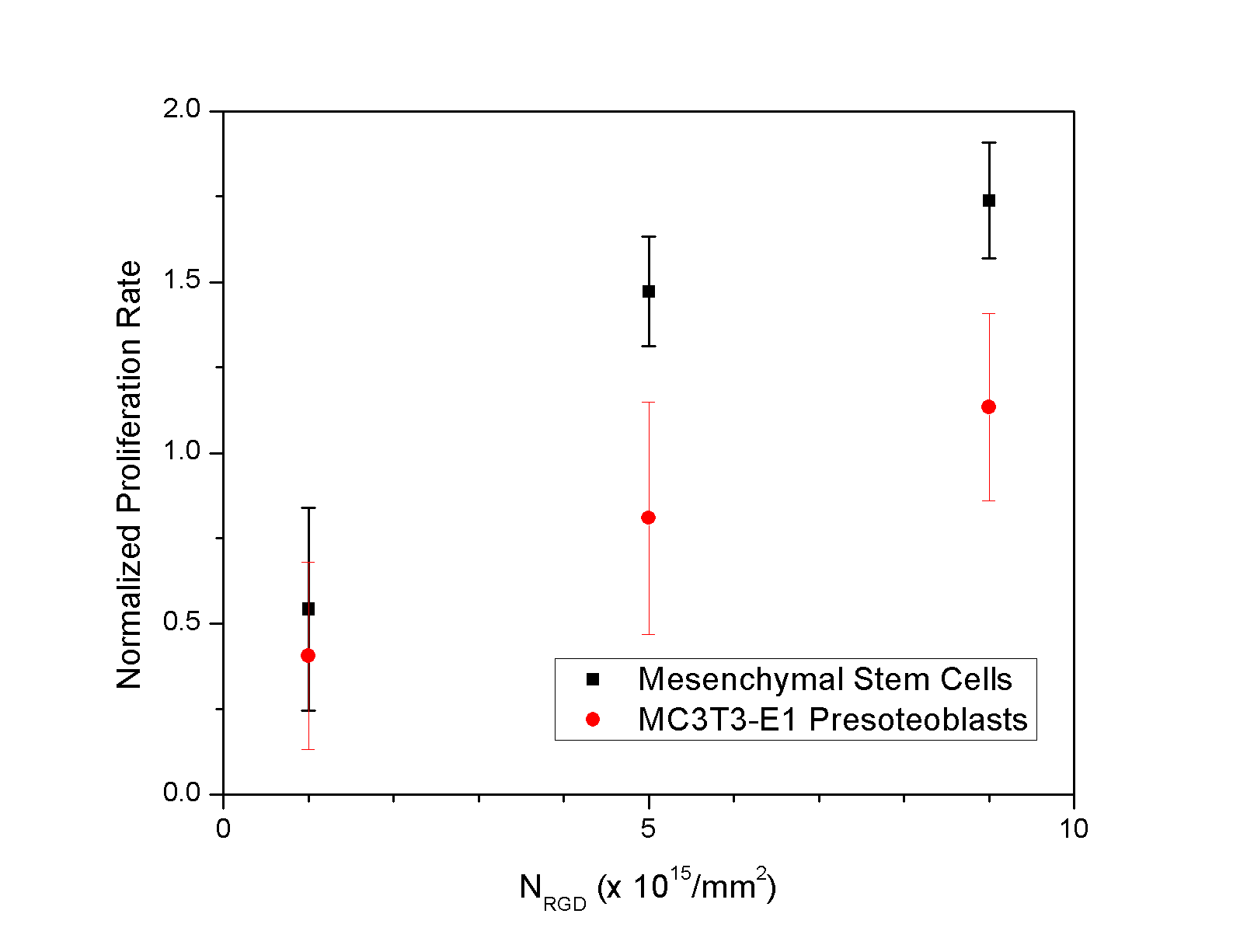
One of the critical components for successfully using stem and progenitor cells in clinical treatments is a large-scale cell culture technology, which regulates the diverse cellular activities (i.e. growth, death, and differentiation) in a sophisticated manner. Currently, dextran and collagen-coated dextran microcarriers are used as the cell adhesion substrates on an industrial scale, because they significantly increase the ratio of the surface area for cell adhesion to the volume of bioreactors. However, currently commercialized microcarriers present several limitations, such as the use of organic solvents in preparation of microcarriers, limited control of biological function, and difficulty in the cell isolation from the microcarriers following the completion of cell culture[1]. Previously, we have demonstrated that chemical and mechanical properties of calcium cross-linked polysaccharide hydrogel modified with oligopeptides containing Arg-Gly-Asp sequence (RGD peptides) is able to regulate proliferation rate and differentiation level of cells adhered to the hydrogel disk surface [2,3]. We hypothesized that processing of these calcium cross-linked alginate hydrogels as microgels would allow us to control the cell proliferation and differentiation on a large scale and readily collect the cells from the microgels through the dissolution of gel matrices in aqueous environment. This hypothesis was examined with RGD peptides-presenting calcium cross-linked alginate microgels with diameters ranging from 300 to 700 mm. The density of RGD peptides was varied by mixing unmodified alginate molecules and RGD peptides-presenting alginate molecules at different ratios. The mechanical stiffness of the gel was controlled with the cross-linking density. We tested both bone precursor cells (MC3T3-E1 presoteoblasts) and bone marrow-derived mesenchymal stem cells. We found that the proliferation rate, shown in Figure 1, and the differentiation level of the cells were significantly controlled with these material variables on a large scale. In addition, a process to collect the expanded cells through the dissolution of microgel matrices with citric acid did not affect cell viability. Overall, the results of this study present a unique cell culture device, which allows one to control the proliferation rate, differentiation level and cell recovery on a large scale. The results of this study will be widely useful in culturing a broad array of stem and progenitor cells within a bioreactor with limited volume and thus greatly benefit the use of cells in various therapeutic interventions.
Figure 1: Cell proliferation was regulated by the overall density of RGD peptides on the alginate microcarriers. The cell proliferation rate was hastened with the increase in the number of RGD peptides (NRGD). Values represent the mean and standard deviation from four independent measurements. 1. Malda, J., & Frondoza, C.G. Microcarriers in the engineering of cartilage and bone. TRENDS in Biotechnology 24:299-304 (2006) 2. Kong, H. J., Boontheekul, T., & Mooney, D. J. Quantifying the relation between adhesion ligand-receptor bond formation and cell phenotype. Proceedings of the 3. Kong, H. J., Liu, J., Riddle, K., Matsumoto, T., Leach, K., & Mooney D.J. Gene delivery regulated by substrate rigidity Nature Materials 4:460 (2005)