768c Electrochemical Performance of Direct Carbon Fuel Cell Based on Molten Carbonate Eutectic Electrolytes
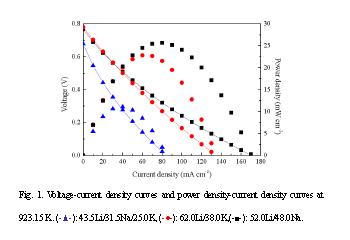
Direct carbon fuel cell (DCFC) has received much attention over the past decade. It represents a way to efficiently convert the chemical energy in solid carbon fuels (mainly from fossil fuels) directly into electrical energy and produce high concentration carbon dioxide that can be easily captured and sequestered. In this process, the thermal efficiency is as high as 100% because of the almost zero entropy change. Molten carbonate is employed as one kind of candidate electrolyte in DCFC in previous study. In this work, the electrochemical performance of a DCFC single cell being used three kinds of molten carbonate eutectic electrolyte with different composition was investigated. Anode fuel: graphite rod, diameter = 6.0 mm; Cathode: closed-bottom porous nickel tube, inner diameter = 41.6 mm, thickness = 4.0 mm, a mixture of CO2 and O2 (with the mol ratio of 2:1) was served as reactant in the cathode; Electrolyte: three kinds of carbonate melt were used, the property of them are listed in table 1; Operating temperature: 923.15 K. The electrochemical performance was measured by Computerized Electrochemical Instruments CBI660B (Chenhua Ltd. Shanghai). Fig. 1 shows the electrochemical performance of the single DCFC cell based on three kinds of molten carbonate electrolytes. The performance of 52.0Li/48.0Na electrolyte is as same as that of 62.0Li/38.0K electrolyte, and both are better than that of 43.5Li/31.5Na/25.0K electrolyte at low current density. However, at high current density, 52.0Li/48.0Na electrolyte performances best among them. The peak power densities are 10.29 mW cm-2, 22.65 mW cm-2, 25.66 mW cm-2 in the order of 43.5Li/31.5Na/25.0K, 62.0Li/38.0K, 52.0Li/48.0Na carbonate electrolyte. In conclusion, molten carbonate is a kind of promising electrolyte in DCFC. For various carbonates with different composition, 52.0Li/48.0Na carbonate melt has the best electrochemical performance. Key words: direct carbon fuel cell; molten carbonates; carbon *Corresponding author, Tel.:+1-734-936-2015, Fax: +1-734-763-0459, E-mail: ltt@umich.edu (Levi T. Thompson) **Corresponding author, Tel.:+86-22-27405613, Fax: +86-22-27405624, E-mail: ydli@tju.edu.cn (Y.D. Li)
Web Page: www.engin.umich.edu/dept/cheme/people/thompson.html and http://ydli.net/