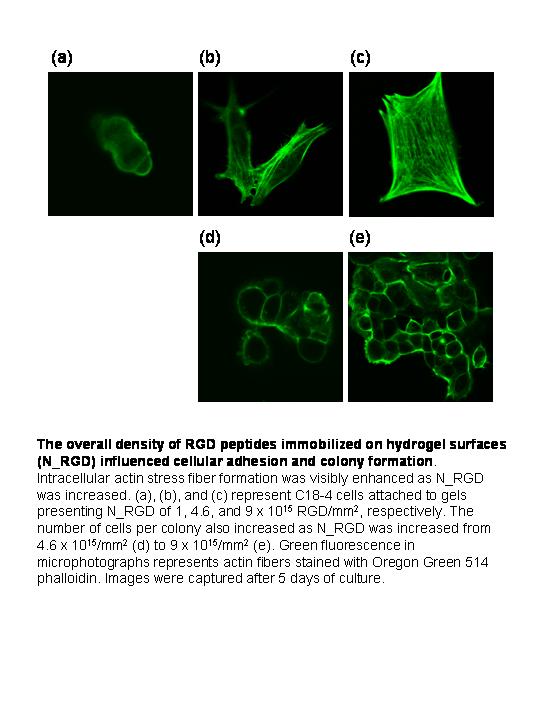
446f Synthetic Stem Cell Niche for In Vitro Spermatogonial Stem Cell Culture
Stem cells are increasingly studied for potential use in various therapies including tissue regeneration. Specifically, spermatogonial stem cells (SSCs) are attracting attention due to their potential pluripotency arising from their ability to dedifferentiate into embryonic stem cell-like cells [1]. For their successful therapeutic use, these cells first must be expanded in vitro using an appropriate culture system. We have previously shown that the mechanical stiffness and cell adhesion cues of hydrogel substrates can be engineered to control cell proliferation [2]. Therefore, we hypothesized that a hydrogel with proper biochemical and biomechanical properties may mimic the composition and structure of the native basement membrane onto which SSCs reside, thus allowing us to control SSC proliferation. This hypothesis was examined with a hydrogel containing chemically linked synthetic oligopeptides including the Arg-Gly-Asp sequence (RGD peptides). The RGD peptide density (N_RGD) and the mechanical stiffness of the hydrogel were varied to examine their effects on cell proliferation in both 2D and 3D cultures. Interestingly, cell proliferation in 2D cultures was greatly dependent on N_RGD but minimally influenced by mechanical stiffness. The N_RGD also modulated the number and size of SSC colonies formed in 3D cultures. Overall, the results of this study elucidate an important factor regulating SSC proliferation and also present a bioactive hydrogel that can be used as a 3D synthetic basement membrane. In addition, the results of this study will be broadly useful in controlling the proliferation of various stem cells and expedite the use of stem cells in clinical treatments. References: [1] Kanatsu-Shinohara, M., Inoue, K., Lee, J., Yoshimoto, M., Ogonuki, N., Miki, H., Baba, S., Kato, T., Kazuki, Y., Toyokuni, S., Toyoshima, M., Niwa, O., Oshimura, M., Heike, T., Nakahata, T., Ishino, F., Ogura, A., and Shinohara, T. Generation of pluripotent stem cells from neonatal mouse testis. Cell 119, 1001, 2004. [2] Kong, H.J., Boontheekul, T., and Mooney, D. J. Quantifying the relation between adhesion ligand-receptor bond formation and cell phenotype. Proc Natl Acad Sci USA 103, 18534, 2006.