83e Low Temperature Scr Activity Over MnOx/TiO2 Catalysts Prepared by Sol-Gel Method; Effect of Mn Loadings
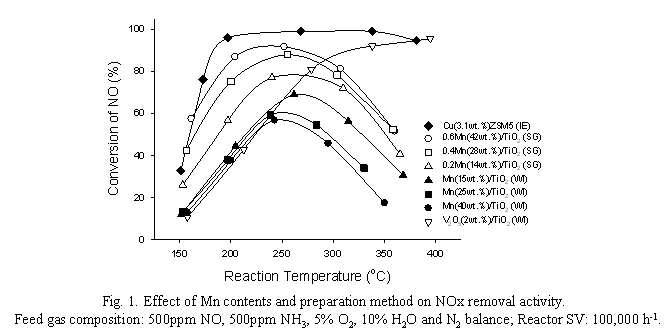
The advent of highly energy-efficient automotive engine including diesel and lean-burn engines for reducing fuel consumption and CO2 emissions has hardly resolved the NOx emission problem yet, mainly due to the requirement of the oxygen-rich feed gas stream to the engines [1,2]. The Selective Catalytic Reduction (SCR) by urea (NH3) is a proven and unique technology to meet the ever stringent air pollution regulations including EURO V and SULEV. The CuZSM5 and V2O5/TiO2 suggested to be effective for the SCR reaction are commonly recognized as an appropriate catalyst. However, these catalysts still struggle from their low temperature activity and the environmental perception of the catalyst composition, copper and vanadium employed onto the catalyst. To resolve these issues, a new catalyst formulation, MnOx/TiO2 catalyst prepared by sol-gel method has been developed in the present study. The MnOx-based catalyst has been suggested as a low temperature SCR catalyst [3,4]. Recently, it had been commercially applied to the stationary source of NOx in the low temperature region from 80 to 250oC [5-7]. Particularly, MnOx/TiO2 has been reported as a best catalytic system revealing the high deNOx performance at low temperature under the feed gas stream without H2O. However, the catalyst had been examined under dry condition without water and at the low reactor space velocity, 8,000 ~ 30,000 hr-1. It has been recognized that water may shift the trend of NOx removal activity to the higher reaction temperature region [3,7]. In addition, the comparative studies for the deNOx performance of the commercial SCR catalysts are commonly conducted at the reactor space velocity of 50,000 ~ 100,000 hr-1 [2]. The purpose of the present study is to develop an effective low temperature SCR catalyst, mainly MnOx/TiO2 catalyst for its commercial application, particularly automotive engine under the realistic reactor operating condition with respect to the catalyst preparation methods, sol-gel (SG) and wet impregnation (WI). The SG method employed is specific and unique compared to the previous method reported [6]. Optimizing the sequence for mixing the catalyst precursors including Mn and TiO2 and solvents, the BET surface area of the catalysts prepared increases to 120 m2/g compared to 75, the highest area reported [6]. The deNOx activity has been examined over a packed-bed flow reactor system at the reactor space velocity of 100,000 hr-1 for its application to automotive engine under the reaction condition of 500ppm NO, 500ppm NH3, 5% O2, 10% H2O and N2 balance. Fig. 1 shows the deNOx activities of MnOx/TiO2 catalysts prepared by SG and WI methods with respect to the catalyst compositions including the molar ratios of Mn/Ti and/or Mn loadings. The NO removal activity of the sol-gel prepared catalysts increases as the catalyst Mn loadings increase. However, the catalyst containing Mn/Ti ratio of 0.6, reveals the highest NO conversion of 93 % at 225oC in the present study. Note that 90 % of NO conversion had been reported over the catalyst containing the Mn/Ti ratio of 0.4 at 150oC and 30,000 hr-1 under the feed gas stream without water by Wu et al. [6]. The catalyst can achieve the NOx conversion of 60 % at about 150oC, the highest activity compared to the previous works reported under the wet gas stream at 100,000 hr-1 as shown in Fig. 1. It may be due to the increase of BET surface area of the catalyst, 120 m2/g prepared by the catalyst preparation method employed in the present study. On the other hand, NO2 and N2O have formed during the course of the SCR reaction over the MnOx/TiO2 catalysts, particularly in the high reaction temperature region above 250oC. It may be probably due to the NH3 oxidation and NO combination (2NO → N2O + O) reactions [8]. The NOx conversion of the catalyst prepared by WI method decreases as the Mn loadings of the catalysts increase. Qi and Yang [7] reported that the amorphous phase of manganese oxide was the active reaction site for the SCR reaction, while the crystalline MnOx reduced the deNOx activity. The metal particles formed on the surface of the catalyst prepared by WI method are easily aggregated as the catalyst Mn contents increase. To identify the active reaction site of MnOx/TiO2 catalysts prepared by sol-gel method in the present study, the catalysts have been characterized by using XRD and In-situ FTIR. No MnOx crystal peaks over the SG prepared catalysts have been observed by XRD, regardless of the Mn contents, whereas the formation of the crystal form of MnOx is apparent over the WI catalysts. Although the Lewis acid sites have been formed over both catalysts confirmed by FTIR, regardless of the catalyst preparation methods, the BrΦnsted acid sites are exclusively observed over the catalysts prepared by the SG method employed in the present study. It has been commonly recognized that the BrΦnsted acid sites play an important role for the SCR reaction, particularly over the zeolite type catalytic systems [9]. TPR and XANES were also employed to elucidate the high performance of the MnOx /TiO2 catalytic systems developed in the present study. In addition, the effect of the NO2/NOx feed ratios on the deNOx performance will be also discussed. References [1] W.S. Epling, L.E. Campbell, A. Yezerets, N.W. Currier, E.P. James, Catal. Rev. 46 (2004) 163. [2] R.M. Heck, R.J. Farrauto, Catalytic Air Pollution Control: Commercial Technology, Van Nostrand Reinhold, New York, 1995. [3] P.G. Smirniotis, D. Pena, B. S. Uphade, Angew. Chem. Int. Ed., 40 (2001) 2479. [4] X. Tang, J. Hao, H. Yi, J. Li, Catal. Today 126 (2007) 406. [5] D.A. Pena, B.S. Uphade, P.G. Smirniotis, J. Catal. 221 (2004) 421. [6] Z. Wu, B. Jiang, Y. Liu, W. Zhao, B. Guan, J. Haz. Mat., 145 (2007) 488. [7] G. Qi, R.T. Yang, Appl. Catal. B:Environ. 44 (2003) 217. [8] J.A. Martin, M. Yates, P. Avila, S. Suarez, J. Blanco, Appl. Catal. B:Environ. 70 (2007) 330. [9] E.Y. Choi, I.-S. Nam, Y.G. Kim, J. Catal., 161 (1996) 597.