238g Effect of Structural Heterogeneity on Adsorption of Water In Microporous Carbons: Insight from Single-Walled Carbon Nanotubes
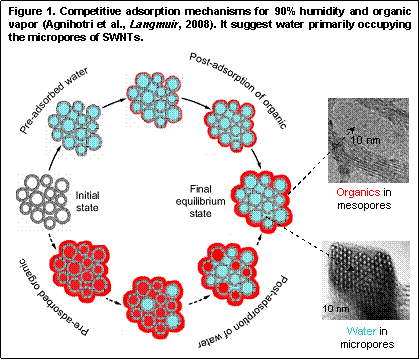
Single-walled carbon nanotubes (SWNTs) have a structure that exhibits greater order but in essence they are microporous activated carbons. While large-scale applications of carbon nanotubes as adsorbent materials are being debated, the structural simplicity of single-walled carbon nanotubes allows them to be studied with a greater degree of accuracy. Therefore, the knowledge gathered from a rigorous joint experimental and theoretical investigation of adsorption processes in single-walled carbon nanotubes should be transferable to those in microporous activated carbons. We have developed a practical approach for adsorption modeling of heterogeneity of SWNTs. The method integrates experimental analysis with grand canonical Monte Carlo (GCMC) simulation of a small probe molecule, such as nitrogen at 77 K. Using this method it is possible to separately estimate adsorption inside the nanotubes, adsorption on the external surface of the bundles, and adsorptive contributions from the impurities present in samples (Agnihotri et al., J Phys Chem C, 2007). Using this methodology, we recently conducted a study on competitive adsorption of water and organic vapors to elucidate the distinct interactions between the select adsorbates and the nanoporous structure of SWNTs (Agnihotri et al, Langmuir, 2008). Experimental adsorption isotherms on SWNT samples for hexane, methyl ethyl ketone, cyclohexane and toluene individually mixed in carrier gases that were nearly saturated with water vapor were compared with the GCMC simulated isotherms for hexane, as a representative organic, on the external surface of the heterogeneous SWNT bundles. From the nearly perfect overlap between the experimental and simulated isotherms, it is concluded that until near saturation only the internal pore volume of pristine SWNT bundles fills with water. The adsorption of water vapor on the peripheral surface of the bundles remains insignificant, if not negligible, in comparison to the adsorption of water in the internal volume of the bundles. This is in contrast with the adsorption of pure hexane, which exhibits appreciable adsorption both inside the bundles as well as on their external surface. It is also suggested that during competitive adsorption, water molecules take precedence over small non-polar and polar organic molecules for adsorption inside SWNTs and leave unoccupied the hydrophobic external surface of the bundles for other more compatible adsorbates. We notice that the internal volume of nanotubes and the void space between the bundles can be broadly categorized as the microporosity and mesoporosity, respectively, of SWNT samples. Therefore, our study suggests that the structural simplicity of SWNTs may be exploited to develop a broader understanding of water adsorption in microporous carbons.