31d Separation of Chemicals Produced by Acidogenic Fermentation
Introduction
Decreasing oil reserves and environmental concerns have motivated the use of alternative and renewable resources, such as biomass, for the production of energy and chemicals. Acidogenic fermentation of biomass for the production of ethanol and acetic, propionic, butyric and lactic acids offers three main advantages: (i) germ free conditions are not required; (ii) very diversified, less pretreated and cheap biomass can be used; (iii) the molecules produced and their esters are consumed on a large scale by industries.
Whereas many studies address the transformation of biomass, few report the product recovery, separation and concentration steps downstream of the biomass transformation. These steps remain, in fact, a major challenge for the expansion of green chemistry in a sustainable development context and in particular for the application of acidogenic fermentation which produces very dilute (concentrations of only a few percent) and complex aqueous solutions of acids and ethanol.
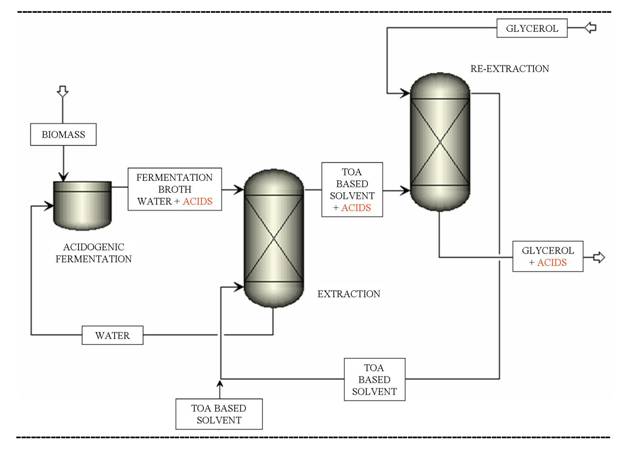
A number of separation processes can be potentially used and the process which is technically, energetically and environmentally the most efficient has not been identified or developed yet. In the present work, the separation step following the acidogenic fermentation of biomass is investigated. A novel extraction - re-extraction process is presented for the recovery of acids and ethanol produced by acidogenic fermentation. The process is based on the transfer of the acids and ethanol to a glycerol phase via an intermediate solvent phase and it is schematically presented in Figure 1. Tri-n-octylamine based solvents are chosen in the first step of the process for their preferential extraction of acids. In the second, re-extraction step, the acids are extracted from the intermediate solvent with glycerol. Glycerol being a by-product of the growing biodiesel production, its offer largely exceeds its demand and its market value has decreased over the years. New markets and applications of glycerol are expected to increase its value1 as well as the profitability of the biodiesel industry. The novel glycerol based extraction – re-extraction process for the recovery of acids opens perspectives for the transformation of glycerol into short chain esters, directly using the acids recovered.
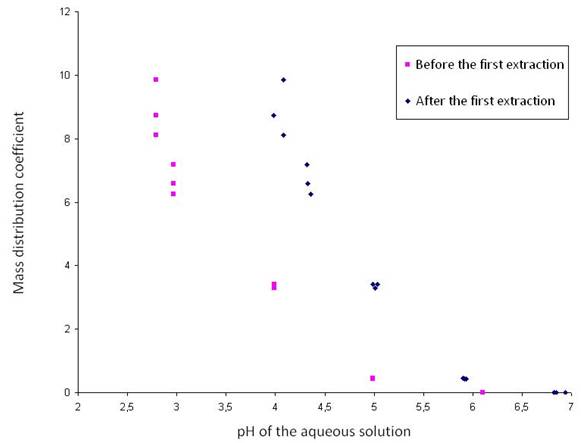
Figure 1: TOA-based extraction – glycerol-based re-extraction process for the separation of volatile fatty acids and ethanol produced by acidogenic fermentation of biomass Experimental set-up and results Experiments were carried out in order to obtain mass based distribution coefficients. Pure tri-n-octylamine (Acros, 98%) was used as intermediate solvent. First, butyric acid aqueous model solutions were tested. Then, the behavior with real fermentation broths was measured. Solutions were stirred to reach equilibrium and then centrifuged. The concentrations of butyric acid in the aqueous phase before and after extraction were determined by gas chromatography. Before the extraction, the pH was not adjusted except when measuring the influence of the pH on the distribution coefficients. The extraction – re-extraction performance was shown to depend highly on the operating conditions. In particular, the influence of the pH, the temperature and the composition of the aqueous phase was studied. The distribution coefficient of butyric acid for the first extraction as a function of the pH of the aqueous solution is presented in Figure 2. The mass distribution coefficient is seen to increase significantly with decreasing pH. Moreover, the pH after the first extraction is linearly related to the pH before the first extraction. Figure 2: The mass distribution coefficient as a function of the pH of the aqueous solution before and after the first extraction For the first extraction step (water/TOA system), the distribution coefficient was shown to increase when decreasing temperature. Inversely, for the second extraction step (TOA/glycerol system), the extraction was more efficient when the temperature increased. The simultaneous presence of butyric and acetic acids did not affect the extraction of the individual acids. Conclusion A novel TOA-based extraction – glycerol-based re-extraction process for the recovery of ethanol and acetic, propionic, butyric and lactic acids from the fermentation broth was experimentally studied. First, the distribution coefficients in simple cases were determined. Next, more complex solutions, approaching fermentation broths, were studied. The extraction re-extraction performance was shown to depend highly on the solvent and on the operating conditions. The influence of the most important process parameters, such as the pH, the temperature, the composition of the aqueous phase was analyzed. The influence of the composition of the organic phase, i.e. different modifiers and diluents, is to be further investigated. Acknowledgements The authors would like to acknowledge the Belgian Fonds pour la formation à la Recherche dans l'Industrie et dans l'Agriculture (F.R.I.A.) for the financial support. References 1. C. O'Driscoll (2007) Biofuels, Bioprod., Bioref., 1, 6–7